Harnessing the Electric Spark of Life to Cure Skin Wounds
- PMID: 24761353
- PMCID: PMC3928811
- DOI: 10.1089/wound.2013.0451
Harnessing the Electric Spark of Life to Cure Skin Wounds
Abstract
Significance: Skin wounds cause great distress and are a huge economic burden, particularly with an increasingly aging population that heals poorly. There is an urgent need for better therapies that improve repair. Intracellular signaling pathways that regulate wound repair are activated by growth factors, hormones, and cytokines released at the wound. In addition, endogenous electric fields (EFs) are generated by epithelia in response to injury and are an important cue that coordinates cell behavior at wounds. Electrical stimulation (ES), therefore, holds the potential to be effective therapeutically in treating wounds. Recent Advances: ES of wounds is an old idea based on observations of the natural occurrence of EF at wound sites. However, it is now receiving increasing attention, because (1) the underpinning mechanisms are being clarified; (2) devices that measure skin wound currents are in place; and (3) medical devices that apply EF to poorly healing wounds are in clinical use with promising results. Critical Issues: Several signaling proteins transduce the EF influence to cells. However, a bigger picture of the EF-proteome is needed in order to understand this complex process and target it in a controlled manner. Future Directions: Dissecting the signaling pathways driving electrical wound healing will allow further identification of key molecular switches that control the cellular response to EFs. These findings herald the development of a new concept, the use of hydrogel electrodes impregnated with small molecules that target signaling pathways to explore the potential of dual electric-pharmacological therapies to repair wounds.
Figures


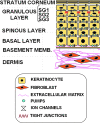
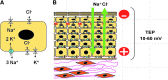
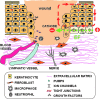

References
-
- Gurtner GC, Werner S, Barrandon Y, and Longaker MT: Wound repair and regeneration. Nature 2008; 453:314. - PubMed
-
- Kloth LC: Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005; 4:23. - PubMed
-
- Ramadan A, Elsaidy M, and Zyada R: Effect of low-intensity direct current on the healing of chronic wounds: a literature review. J Wound Care 2008; 17:292. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

