Chronic brain inflammation causes a reduction in GluN2A and GluN2B subunits of NMDA receptors and an increase in the phosphorylation of mitogen-activated protein kinases in the hippocampus
- PMID: 24761931
- PMCID: PMC4021635
- DOI: 10.1186/1756-6606-7-33
Chronic brain inflammation causes a reduction in GluN2A and GluN2B subunits of NMDA receptors and an increase in the phosphorylation of mitogen-activated protein kinases in the hippocampus
Abstract
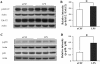
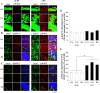
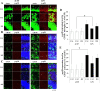
Neuroinflammation plays a key role in the initiation and progression of neurodegeneration in Alzheimer's disease (AD). Chronic neuroinflammation results in diminished synaptic plasticity and loss of GluN1 N-methyl-D-aspartate (NMDA) receptors in the hippocampus, leading to the cognitive deficits that are the most common symptoms of AD. Therefore, it is suggested that chronic inflammation may alter expression levels of GluN2A and GluN2B subunits of NMDA receptors and associated intracellular signalling. Chronic neuroinflammation was induced by chronic infusion of lipopolysaccharide (LPS) into the fourth ventricle in Fischer-344 rats. The status of hippocampus-dependent memory was evaluated in control rats and rats chronically infused with LPS. Microglial activation in the hippocampus was examined using immunohistochemical staining. Western blot analysis was used to measure membrane levels of GluN2A and GluN2B subunits of NMDA receptors and mitogen-activated protein kinase (MAPK) in the hippocampi of these rats, and immunofluorescent double labeling was used to assess the cellular location of MAPK. Microglial activation was observed in the hippocampi of rats that showed memory impairments with chronic LPS infusion. Chronic LPS infusion reduced the levels of GluN2A and GluN2B and increased the levels of phosphorylated MAPKs in the hippocampus. MAPK-positive immunoreactivity was observed mostly in the neurons and also in non-neuronal cells. Reductions in GluN2A and GluN2B subunits of NMDA receptors coupled with altered MAPK signaling, in response to inflammatory stimuli may be related to the cognitive deficits observed in AD.
Figures
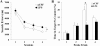
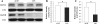
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I. et al.Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. - DOI - PMC - PubMed
-
- McGeer PL, McGeer EG. Inflammation of the brain in Alzheimer’s disease: implications for therapy. J Leukoc Biol. 1999;65:409–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

