Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists
- PMID: 24762001
- PMCID: PMC4128043
- DOI: 10.1111/bph.12744
Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists
Abstract
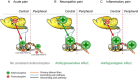
Despite high sequence similarity between NOP (nociceptin/orphanin FQ opioid peptide) and opioid receptors, marked differences in endogenous ligand selectivity, signal transduction, phosphorylation, desensitization, internalization and trafficking have been identified; underscoring the evolutionary difference between NOP and opioid receptors. Activation of NOP receptors affects nociceptive transmission in a site-specific manner, with antinociceptive effects prevailing after peripheral and spinal activation, and pronociceptive effects after supraspinal activation in rodents. The net effect of systemically administered NOP receptor agonists on nociception is proposed to depend on the relative contribution of peripheral, spinal and supraspinal activation, and this may depend on experimental conditions. Functional expression and regulation of NOP receptors at peripheral and central sites of the nociceptive pathway exhibits a high degree of plasticity under conditions of neuropathic and inflammatory pain. In rodents, systemically administered NOP receptor agonists exerted antihypersensitive effects in models of neuropathic and inflammatory pain. However, they were largely ineffective in acute pain while concomitantly evoking severe motor side effects. In contrast, systemic administration of NOP receptor agonists to non-human primates (NHPs) exerted potent and efficacious antinociception in the absence of motor and sedative side effects. The reason for this species difference with respect to antinociceptive efficacy and tolerability is not clear. Moreover, co-activation of NOP and μ-opioid peptide (MOP) receptors synergistically produced antinociception in NHPs. Hence, both selective NOP receptor as well as NOP/MOP receptor agonists may hold potential for clinical use as analgesics effective in conditions of acute and chronic pain.
© 2014 The British Pharmacological Society.
Figures
References
-
- Agostini S, Eutamene H, Broccardo M, Improta G, Petrella C, Theodorou V, et al. Peripheral anti-nociceptive effect of nociceptin/orphanin FQ in inflammation and stress-induced colonic hyperalgesia in rats. Pain. 2009;141:292–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

