Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer
- PMID: 24762066
- PMCID: PMC4053180
- DOI: 10.1186/bcr3648
Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer
Abstract
Introduction: Although neoadjuvant chemotherapy (NAC) for locally advanced breast cancer can improve operability and local disease control, there is a lack of reliable biomarkers that predict response to chemotherapy or long-term survival. Since expression of aldehyde dehydrogenase-1 (ALDH1) is associated with the stem-like properties of self-renewal and innate chemoresistance in breast cancer, we asked whether expression in serial tumor samples treated with NAC could identify women more likely to benefit from this therapy.
Methods: Women with locally advanced breast cancer were randomly assigned to receive four cycles of anthracycline-based chemotherapy, followed by four cycles of taxane therapy (Arm A), or the same regimen in reverse order (Arm B). Tumor specimens were collected at baseline, after four cycles, and then at surgical resection. ALDH1 expression was determined by immunohistochemistry and correlated with tumor response using Fisher's exact test while Kaplan-Meier method was used to calculate survival.
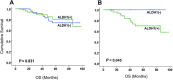
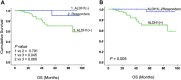
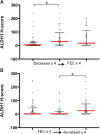
Results: A hundred and nineteen women were enrolled into the study. Fifty seven (48%) were randomized to Arm A and 62 (52%) to Arm B. Most of the women (90%) had ductal carcinoma and 10% had lobular carcinoma. Of these, 26 (22%) achieved a pathological complete response (pCR) after NAC. There was no correlation between baseline ALDH1 expression and tumor grade, stage, hormone receptor, human epidermal growth factor receptor 2 (HER2) status and Ki67 index. ALDH1 negativity at baseline was significantly associated with pCR (P = 0.004). The presence of ALDH1(+) cells in the residual tumor cells in non-responding women was strongly predictive of worse overall survival (P = 0.024). Moreover, serial analysis of specimens from non-responders showed a marked increase in tumor-specific ALDH1 expression (P = 0.028). Overall, there was no survival difference according to the chemotherapy sequence. However, poorly responding tumours from women receiving docetaxel chemotherapy showed an unexpected significant increase in ALDH1 expression.
Conclusions: ALDH1 expression is a useful predictor of chemoresistance. The up-regulation of ALDH1 after NAC predicts poor survival in locally advanced breast cancer. Although the chemotherapy sequence had no effect on overall prognosis, our results suggest that anthracycline-based chemotherapy may be more effective at targeting ALDH1(+) breast cancer cells.
Trial registration: ACTRN12605000588695.
Figures




References
-
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. - PubMed
-
- Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, Fellin FM, McFarlane J. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–369. doi: 10.1002/1097-0142(19940115)73:2<362::AID-CNCR2820730221>3.0.CO;2-L. - DOI - PubMed
-
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
-
- Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, Hudis C, Gray JW, Perou C, Yau C, Livasy C, Krontiras H, Montgomery L, Tripathy D, Lehman C, Liu MC, Olopade OI, Rugo HS, Carpenter JT, Dressler L, Chhieng D, Singh B, Mies C, Rabban J, Chen YY, Giri D, Van ’t Veer L, Hylton N. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

