Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo
- PMID: 24762191
- PMCID: PMC4113202
- DOI: 10.1186/1465-9921-15-51
Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a progressive disease of insidious onset, and is responsible for up to 30,000 deaths per year in the U.S. Excessive production of extracellular matrix by myofibroblasts has been shown to be an important pathological feature in IPF. TGF-β1 is expressed in fibrotic lung and promotes fibroblast to myofibroblast differentiation (FMD) as well as matrix deposition.
Methods: To identify the mechanism of Arsenic trioxide's (ATO)'s anti-fibrotic effect in vitro, normal human lung fibroblasts (NHLFs) were treated with ATO for 24 hours and were then exposed to TGF-β1 (1 ng/ml) before harvesting at multiple time points. To investigate whether ATO is able to alleviate lung fibrosis in vivo, C57BL/6 mice were administered bleomycin by oropharyngeal aspiration and ATO was injected intraperitoneally daily for 14 days. Quantitative real-time PCR, western blotting, and immunofluorescent staining were used to assess the expression of fibrotic markers such as α-smooth muscle actin (α-SMA) and α-1 type I collagen.
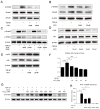
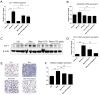
Results: Treatment of NHLFs with ATO at very low concentrations (10-20nM) inhibits TGF-β1-induced α-smooth muscle actin (α-SMA) and α-1 type I collagen mRNA and protein expression. ATO also diminishes the TGF-β1-mediated contractile response in NHLFs. ATO's down-regulation of profibrotic molecules is associated with inhibition of Akt, as well as Smad2/Smad3 phosphorylation. TGF-β1-induced H2O2 and NOX-4 mRNA expression are also blocked by ATO. ATO-mediated reduction in Smad3 phosphorylation correlated with a reduction of promyelocytic leukemia (PML) nuclear bodies and PML protein expression. PML-/- mouse embryonic fibroblasts (MEFs) showed decreased fibronectin and PAI-1 expression in response to TGF-β1. Daily intraperitoneal injection of ATO (1 mg/kg) in C57BL/6 mice inhibits bleomycin induced lung α-1 type I collagen mRNA and protein expression.
Conclusions: In summary, these data indicate that low concentrations of ATO inhibit TGF-β1-induced fibroblast to myofibroblast differentiation and decreases bleomycin induced pulmonary fibrosis.
Figures





Similar articles
-
Sequential analysis of myofibroblast differentiation and transforming growth factor-β1/Smad pathway activation in murine pulmonary fibrosis.J Nippon Med Sch. 2012;79(1):46-59. doi: 10.1272/jnms.79.46. J Nippon Med Sch. 2012. PMID: 22398790
-
USP7 Promotes TGF-β1 Signaling by De-Ubiquitinating Smad2/Smad3 in Pulmonary Fibrosis.Discov Med. 2024 Aug;36(187):1616-1626. doi: 10.24976/Discov.Med.202436187.148. Discov Med. 2024. PMID: 39190377
-
Curdione ameliorates bleomycin-induced pulmonary fibrosis by repressing TGF-β-induced fibroblast to myofibroblast differentiation.Respir Res. 2020 Feb 19;21(1):58. doi: 10.1186/s12931-020-1300-y. Respir Res. 2020. PMID: 32075634 Free PMC article.
-
METTL3-mediated m6A RNA methylation induces the differentiation of lung resident mesenchymal stem cells into myofibroblasts via the miR-21/PTEN pathway.Respir Res. 2023 Nov 28;24(1):300. doi: 10.1186/s12931-023-02606-z. Respir Res. 2023. PMID: 38017523 Free PMC article. Review.
-
Matrix abnormalities in pulmonary fibrosis.Eur Respir Rev. 2018 Jun 27;27(148):180033. doi: 10.1183/16000617.0033-2018. Print 2018 Jun 30. Eur Respir Rev. 2018. PMID: 29950306 Free PMC article. Review.
Cited by
-
N-acetylcysteine attenuates sodium arsenite-induced oxidative stress and apoptosis in embryonic fibroblast cells.Toxicol Res (Camb). 2024 Aug 13;13(4):tfae128. doi: 10.1093/toxres/tfae128. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39139367 Free PMC article.
-
Low-dose arsenic trioxide inhibits pancreatic stellate cell activation via LOXL3 expression to enhance immunotherapy in pancreatic cancer.Br J Cancer. 2024 Dec;131(12):1928-1941. doi: 10.1038/s41416-024-02880-8. Epub 2024 Nov 5. Br J Cancer. 2024. PMID: 39501090
-
Fibroblast-to-myofibroblast transition in bronchial asthma.Cell Mol Life Sci. 2018 Nov;75(21):3943-3961. doi: 10.1007/s00018-018-2899-4. Epub 2018 Aug 12. Cell Mol Life Sci. 2018. PMID: 30101406 Free PMC article. Review.
-
Drug delivery systems for wound healing treatment of upper airway injury.Expert Opin Drug Deliv. 2024 Apr;21(4):573-591. doi: 10.1080/17425247.2024.2340653. Epub 2024 Apr 10. Expert Opin Drug Deliv. 2024. PMID: 38588553 Free PMC article. Review.
-
Celastrol attenuates ventilator induced lung injury in mouse through inhibition of MAPK pathway.Int J Clin Exp Pathol. 2017 Sep 1;10(9):9302-9309. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966802 Free PMC article.
References
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810Y816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

