Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment
- PMID: 24762207
- PMCID: PMC4175025
- DOI: 10.1089/ars.2014.5941
Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment
Abstract
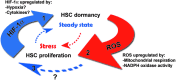
Significance: Blood forming, hematopoietic stem cells (HSCs) mostly reside in the bone marrow in a quiescent, nonmotile state via adhesion interactions with stromal cells and macrophages. Quiescent, proliferating, and differentiating stem cells have different metabolism, and accordingly different amounts of intracellular reactive oxygen species (ROS). Importantly, ROS is not just a byproduct of metabolism, but also plays a role in stem cell state and function.
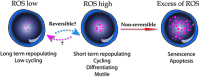
Recent advances: ROS levels are dynamic and reversibly dictate enhanced cycling and myeloid bias in ROS(high) short-term repopulating stem cells, and ROS(low) quiescent long-term repopulating stem cells. Low levels of ROS, regulated by intrinsic factors such as cell respiration or nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) activity, or extrinsic factors such as stem cell factor or prostaglandin E2 are required for maintaining stem cell self-renewal. High ROS levels, due to stress and inflammation, induce stem cell differentiation and enhanced motility.
Critical issues: Stem cells need to be protected from high ROS levels to avoid stem cell exhaustion, insufficient host immunity, and leukemic transformation that may occur during chronic inflammation. However, continuous low ROS production will lead to lack of stem cell function and opportunistic infections. Ultimately, balanced ROS levels are crucial for maintaining the small stem cell pool and host immunity, both in homeostasis and during stress situations.
Future directions: Deciphering the signaling pathway of ROS in HSC will provide a better understanding of ROS roles in switching HSC from quiescence to activation and vice versa, and will also shed light on the possible roles of ROS in leukemia initiation and development.
Figures


References
-
- Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, and Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem 274: 23916–23925, 1999 - PubMed
-
- Byun HO, Jung HJ, Seo YH, Lee YK, Hwang SC, Hwang ES, and Yoon G. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor (TGF) beta1-induced senescence. Exp Cell Res 318: 1808–1819, 2012 - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, and Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

