Inhibitors - cellular aspects and novel approaches for tolerance
- PMID: 24762281
- PMCID: PMC4205104
- DOI: 10.1111/hae.12407
Inhibitors - cellular aspects and novel approaches for tolerance
Abstract
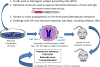
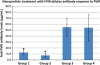
The immune response against therapeutic clotting factors VIII and IX (FVIII and FIX) is a major adverse event that can effectively thwart their effectiveness in correcting bleeding disorders. Thus, a significant number of haemophilia patients form antibodies, called inhibitors, which neutralize the procoagulant functions of therapeutic cofactors FVIII (haemophilia A) or FIX (haemophilia B). Understanding the cellular and molecular aspects of inhibitor formation is critical to designing tolerogenic therapies for clinical use. This review will focus on the basis of the immune response to FVIII, in particular, and will discuss emerging efforts to not only reduce immunogenicity but also to prevent and/or reverse inhibitor formation.
Keywords: factor VIII; gene therapy; haemophilia; immunoglobulin fusions; regulatory T cells.
© 2014 John Wiley & Sons Ltd.
Figures

Similar articles
-
Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors Organization.Br J Haematol. 2013 Jan;160(2):153-70. doi: 10.1111/bjh.12091. Epub 2012 Nov 15. Br J Haematol. 2013. PMID: 23157203 No abstract available.
-
The management of hemophilia patients with inhibitors.Transfus Med Rev. 1992 Oct;6(4):285-93. doi: 10.1016/s0887-7963(92)70185-0. Transfus Med Rev. 1992. PMID: 1421831 Review. No abstract available.
-
Key issues in inhibitor management in patients with haemophilia.Blood Transfus. 2014 Jan;12 Suppl 1(Suppl 1):s319-29. doi: 10.2450/2013.0246-12. Epub 2013 Dec 3. Blood Transfus. 2014. PMID: 24333092 Free PMC article. Review. No abstract available.
-
Switching haemophilia products and inhibitor risk: a United States' perspective.Eur J Haematol. 2015 Apr;94(4):283. doi: 10.1111/ejh.12441. Eur J Haematol. 2015. PMID: 25800968 No abstract available.
-
The molecular basis of haemophilia A and B.Baillieres Clin Haematol. 1996 Jun;9(2):211-28. doi: 10.1016/s0950-3536(96)80059-x. Baillieres Clin Haematol. 1996. PMID: 8800501 Review.
Cited by
-
An Observational Study from Long-Term AAV Re-administration in Two Hemophilia Dogs.Mol Ther Methods Clin Dev. 2018 Aug 4;10:257-267. doi: 10.1016/j.omtm.2018.07.011. eCollection 2018 Sep 21. Mol Ther Methods Clin Dev. 2018. PMID: 30140713 Free PMC article.
-
Factor VIII: Perspectives on Immunogenicity and Tolerogenic Strategies for Hemophilia A Patients.Int J Mol Cell Med. 2020 Winter;9(1):33-50. doi: 10.22088/IJMCM.BUMS.9.1.33. Int J Mol Cell Med. 2020. PMID: 32832483 Free PMC article. Review.
-
Escape or Fight: Inhibitors in Hemophilia A.Front Immunol. 2020 Mar 24;11:476. doi: 10.3389/fimmu.2020.00476. eCollection 2020. Front Immunol. 2020. PMID: 32265927 Free PMC article. Review.
-
The 1.7 Å X-ray crystal structure of the porcine factor VIII C2 domain and binding analysis to anti-human C2 domain antibodies and phospholipid surfaces.PLoS One. 2015 Mar 16;10(3):e0122447. doi: 10.1371/journal.pone.0122447. eCollection 2015. PLoS One. 2015. PMID: 25775247 Free PMC article.
-
Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles.Nat Nanotechnol. 2016 Oct;11(10):890-899. doi: 10.1038/nnano.2016.135. Epub 2016 Aug 1. Nat Nanotechnol. 2016. PMID: 27479756
References
-
- Playfair JH. A cellular basis for auto-immunity. Essays Fundam Immunol. 1973:44–56. - PubMed
-
- Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nat Clin Pract Rheumatol. 2006;2:44–52. - PubMed
-
- Aledort LM, Evatt BL, Lusher JM, Brown-stein AP. HIV and hemophilia. J Thromb Haemost. 2007;5:607–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

