Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect
- PMID: 24762441
- PMCID: PMC4038572
- DOI: 10.1172/JCI72434
Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect
Abstract
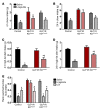
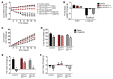
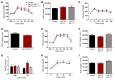
Glucose control and weight loss are cornerstones of type 2 diabetes treatment. Currently, only glucagon-like peptide-1 (GLP1) analogs are able to achieve both weight loss and glucose tolerance. Both glucose and body weight are regulated by the brain, which contains GLP1 receptors (GLP1R). Even though the brain is poised to mediate the effects of GLP1 analogs, it remains unclear whether the glucose- and body weight-lowering effects of long-acting GLP1R agonists are via direct action on CNS GLP1R or the result of downstream activation of afferent neuronal GLP1R. We generated mice with either neuronal or visceral nerve-specific deletion of Glp1r and then administered liraglutide, a long-acting GLP1R agonist. We found that neither reduction of GLP1R in the CNS nor in the visceral nerves resulted in alterations in body weight or food intake in animals fed normal chow or a high-fat diet. Liraglutide treatment provided beneficial glucose-lowering effects in both chow- and high-fat-fed mice lacking GLP1R in the CNS or visceral nerves; however, liraglutide was ineffective at altering food intake, body weight, or causing a conditioned taste aversion in mice lacking neuronal GLP1R. These data indicate that neuronal GLP1Rs mediate body weight and anorectic effects of liraglutide, but are not required for glucose-lowering effects.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

