Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species
- PMID: 24762628
- PMCID: PMC3998915
- DOI: 10.1371/journal.pgen.1004293
Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species
Abstract
Temperature affects both the timing and outcome of animal development, but the detailed effects of temperature on the progress of early development have been poorly characterized. To determine the impact of temperature on the order and timing of events during Drosophila melanogaster embryogenesis, we used time-lapse imaging to track the progress of embryos from shortly after egg laying through hatching at seven precisely maintained temperatures between 17.5 °C and 32.5 °C. We employed a combination of automated and manual annotation to determine when 36 milestones occurred in each embryo. D. melanogaster embryogenesis takes [Formula: see text]33 hours at 17.5 °C, and accelerates with increasing temperature to a low of 16 hours at 27.5 °C, above which embryogenesis slows slightly. Remarkably, while the total time of embryogenesis varies over two fold, the relative timing of events from cellularization through hatching is constant across temperatures. To further explore the relationship between temperature and embryogenesis, we expanded our analysis to cover ten additional Drosophila species of varying climatic origins. Six of these species, like D. melanogaster, are of tropical origin, and embryogenesis time at different temperatures was similar for them all. D. mojavensis, a sub-tropical fly, develops slower than the tropical species at lower temperatures, while D. virilis, a temperate fly, exhibits slower development at all temperatures. The alpine sister species D. persimilis and D. pseudoobscura develop as rapidly as tropical flies at cooler temperatures, but exhibit diminished acceleration above 22.5 °C and have drastically slowed development by 30 °C. Despite ranging from 13 hours for D. erecta at 30 °C to 46 hours for D. virilis at 17.5 °C, the relative timing of events from cellularization through hatching is constant across all species and temperatures examined here, suggesting the existence of a previously unrecognized timer controlling the progress of embryogenesis that has been tuned by natural selection as each species diverges.
Conflict of interest statement
MBE is a cofounder and member of the Board of Directors of PLOS.
Figures



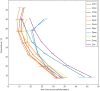
 measured as the mean time between cellularization and trachea fill, has a logarithmic relationship to temperature described by
measured as the mean time between cellularization and trachea fill, has a logarithmic relationship to temperature described by  where T is temperature in °C (
where T is temperature in °C ( ). (C) The developmental rate in D. melanogaster changes uniformly with temperature, not preferentially affecting any stage. Timing here is normalized between the end of cellularization and the filling of the trachea.
). (C) The developmental rate in D. melanogaster changes uniformly with temperature, not preferentially affecting any stage. Timing here is normalized between the end of cellularization and the filling of the trachea.

References
-
- Powsner L (1935) The effects of temperature on the durations of the developmental stages of drosophila melanogaster. Physiological Zoology 8: 474–520.
-
- Yamamoto A, Ohba S (1982) Strategic differences in thermal adaptation between two drosophila species, d. virilis and d. immigrans. Oecologia 52: 333–339. - PubMed
-
- Lillie FR, Knowlton FP (1897) On the effect of temperature on the development of animals. Zoological Bulletin 1: 179–193.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

