Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study
- PMID: 24762631
- PMCID: PMC4017893
- DOI: 10.1093/cid/ciu135
Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study
Abstract
Background: Recurrent Clostridium difficile infection (CDI) with poor response to standard antimicrobial therapy is a growing medical concern. We aimed to investigate the outcomes of fecal microbiota transplant (FMT) for relapsing CDI using a frozen suspension from unrelated donors, comparing colonoscopic and nasogastric tube (NGT) administration.
Methods: Healthy volunteer donors were screened and a frozen fecal suspension was generated. Patients with relapsing/refractory CDI were randomized to receive an infusion of donor stools by colonoscopy or NGT. The primary endpoint was clinical resolution of diarrhea without relapse after 8 weeks. The secondary endpoint was self-reported health score using standardized questionnaires.
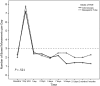
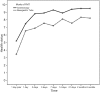
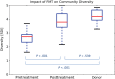
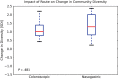
Results: A total of 20 patients were enrolled, 10 in each treatment arm. Patients had a median of 4 (range, 2-16) relapses prior to study enrollment, with 5 (range, 3-15) antibiotic treatment failures. Resolution of diarrhea was achieved in 14 patients (70%) after a single FMT (8 of 10 in the colonoscopy group and 6 of 10 in the NGT group). Five patients were retreated, with 4 obtaining cure, resulting in an overall cure rate of 90%. Daily number of bowel movements changed from a median of 7 (interquartile range [IQR], 5-10) the day prior to FMT to 2 (IQR, 1-2) after the infusion. Self-ranked health score improved significantly, from a median of 4 (IQR, 2-6) before transplant to 8 (IQR, 5-9) after transplant. No serious or unexpected adverse events occurred.
Conclusions: In our initial feasibility study, FMT using a frozen inoculum from unrelated donors is effective in treating relapsing CDI. NGT administration appears to be as effective as colonoscopic administration.
Clinical trials registration: NCT01704937.
Keywords: Clostridium difficile; fecal microbiota transplant; frozen inoculum; microbiome.
Figures
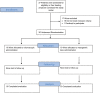
References
-
- Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–70. - PubMed
-
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–40. - PubMed
-
- Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167:567–73. - PubMed
-
- Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

