S-nitrosylation of FLICE inhibitory protein determines its interaction with RIP1 and activation of NF-κB
- PMID: 24762656
- PMCID: PMC4111758
- DOI: 10.4161/cc.28898
S-nitrosylation of FLICE inhibitory protein determines its interaction with RIP1 and activation of NF-κB
Abstract
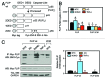
Death receptor (DR) ligation can lead to divergent signaling pathways causing either caspase-mediated cell death or cell proliferation and inflammation. These variations in cellular fate are determined by adaptor proteins that are recruited to the DR signaling complex. FLICE inhibitory protein (FLIP) is an established inhibitor of caspase-8-mediated apoptosis, and it is also involved in NF-κB activation. However, the molecular mechanism that regulates FLIP within this complex is unknown. In this study, we provide new evidence for the regulation of NF-κB by FLIP through S-nitrosylation, which involves covalent modification of the protein's cysteine thiol by nitric oxide to form S-nitrosothiol. Point mutations of FLIP at cysteine residues 254 and 259 prevent FLIP S-nitrosylation and its ability to activate NF-κB. The mechanism by which FLIP nitrosylation regulates NF-κB activity involves RIP1 binding and redistribution, whereas TRAF2 binding and distribution are unaffected. We further show that FLIP processing and cleavage is dependent on its nitrosylation status. Collectively, our study reveals a novel pathway for FLIP regulation of NF-κB through protein S-nitrosylation, which is a key posttranslational mechanism controlling DR-mediated cell death and survival. Since increased expression of FLIP and nitric oxide are frequently observed in chemotherapy-resistant tumors, S-nitrosylation of FLIP could be a key mechanism of chemoresistance and tumor growth.
Keywords: FLIP; NF-κB; RIP1; S-nitrosylation; cancer.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
