Use of a synthetic biosensor for neutralizing activity-biased selection of monoclonal antibodies against atroxlysin-I, an hemorrhagic metalloproteinase from Bothrops atrox snake venom
- PMID: 24762927
- PMCID: PMC3998924
- DOI: 10.1371/journal.pntd.0002826
Use of a synthetic biosensor for neutralizing activity-biased selection of monoclonal antibodies against atroxlysin-I, an hemorrhagic metalloproteinase from Bothrops atrox snake venom
Abstract
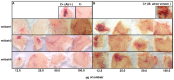
Background: The snake Bothrops atrox is responsible for the majority of envenomings in the northern region of South America. Severe local effects, including hemorrhage, which are mainly caused by snake venom metalloproteinases (SVMPs), are not fully neutralized by conventional serum therapy. Little is known about the immunochemistry of the P-I SVMPs since few monoclonal antibodies (mAbs) against these molecules have been obtained. In addition, producing toxin-neutralizing mAbs remains very challenging.
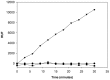
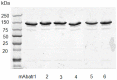
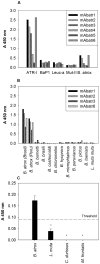
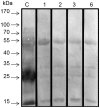
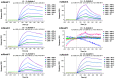
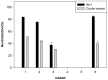
Methodology/principal findings: Here, we report on the set-up of a functional screening based on a synthetic peptide used as a biosensor to select neutralizing mAbs against SVMPs and the successful production of neutralizing mAbs against Atroxlysin-I (Atr-I), a P-I SVMP from B. atrox. Hybridomas producing supernatants with inhibitory effect against the proteolytic activity of Atr-I towards the FRET peptide Abz-LVEALYQ-EDDnp were selected. Six IgG1 Mabs were obtained (named mAbatr1 to mAbatr6) and also two IgM. mAbatrs1, 2, 3 and 6 were purified. All showed a high specific reactivity, recognizing only Atr-I and B. atrox venom in ELISA and a high affinity, showing equilibrium constants in the nM range for Atr-I. These mAbatrs were not able to bind to Atr-I overlapping peptides, suggesting that they recognize conformational epitopes.
Conclusions/significance: For the first time a functional screening based on a synthetic biosensor was successfully used for the selection of neutralizing mAbs against SVMPs.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: The authors Sandra Cobo and Pascale Galéa are employed by the comercial part (BioRad) of the mix enterprise SysDiag. In addition, mAbatrs are in the patent application process. This does not alter our adherence to all PLOS NTDs policies on sharing data and materials.
Figures

References
-
- Williams D, Gutierrez JM, Harrison R, Warrell DA, White J, et al. (2010) The Global Snake Bite Initiative: an antidote for snake bite. Lancet 375: 89–91. - PubMed
-
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância E (2010) Guia de vigilância epidemiológica. Brasília. 816–816 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

