Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial
- PMID: 24762958
- PMCID: PMC4090722
- DOI: 10.1038/bjc.2014.211
Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial
Abstract
Background: The SHIVA trial is a multicentric randomised proof-of-concept phase II trial comparing molecularly targeted therapy based on tumour molecular profiling vs conventional therapy in patients with any type of refractory cancer. RESULTS of the feasibility study on the first 100 enrolled patients are presented.
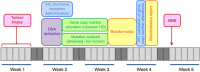
Methods: Adult patients with any type of metastatic cancer who failed standard therapy were eligible for the study. The molecular profile was performed on a mandatory biopsy, and included mutations and gene copy number alteration analyses using high-throughput technologies, as well as the determination of oestrogen, progesterone, and androgen receptors by immunohistochemistry (IHC).
Results: Biopsy was safely performed in 95 of the first 100 included patients. Median time between the biopsy and the therapeutic decision taken during a weekly molecular biology board was 26 days. Mutations, gene copy number alterations, and IHC analyses were successful in 63 (66%), 65 (68%), and 87 (92%) patients, respectively. A druggable molecular abnormality was present in 38 patients (40%).
Conclusions: The establishment of a comprehensive tumour molecular profile was safe, feasible, and compatible with clinical practice in refractory cancer patients.
Figures

References
-
- Andre F, Bachelot T, Campone M, Arnedos M, Dieras V, Lacroix-Triki M, Lazar V, Gentien D, Cohen P, Goncalves A, Lacroix L, Chaffanet M, Dalenc F, Mathieu MC, Bieche I, Olschwang S, Wang Q, Commo F, Jimenez M, Bonnefoi HR.2013Array CGH and DNA sequencing to personalize targeted treatment of metastatic breast cancer (MBC) patients (pts): a prospective multicentric trial (SAFIR01) J Clin Oncol 31(abstr 511).
-
- Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–2696. - PubMed
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. - PubMed
-
- Doroshow JH. Selecting systemic cancer therapy one patient at a time: is there a role for molecular profiling of individual patients with advanced solid tumors. J Clin Oncol. 2010;28:4869–4871. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

