Functional roles of the sweet taste receptor in oral and extraoral tissues
- PMID: 24763065
- PMCID: PMC4059820
- DOI: 10.1097/MCO.0000000000000058
Functional roles of the sweet taste receptor in oral and extraoral tissues
Abstract
Purpose of review: This review summarizes and discusses the current knowledge about the physiological roles of the sweet taste receptor in oral and extraoral tissues.
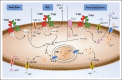
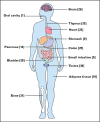
Recent findings: The expression of a functional sweet taste receptor has been reported in numerous extragustatory tissues, including the gut, pancreas, bladder, brain and, more recently, bone and adipose tissues. In the gut, this receptor has been suggested to be involved in luminal glucose sensing, the release of some satiety hormones, the expression of glucose transporters, and the maintenance of glucose homeostasis. More recently, the sweet taste receptor was proposed to regulate adipogenesis and bone biology.
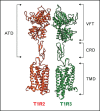
Summary: The perception of sweet taste is mediated by the T1R2/T1R3 receptor, which is expressed in the oral cavity, wherein it provides input on the caloric and macronutrient contents of ingested food. This receptor recognizes all the chemically diverse compounds perceived as sweet by human beings, including natural sugars and sweeteners. Importantly, the expression of a functional sweet taste receptor has been reported in numerous extragustatory tissues, wherein it has been proposed to regulate metabolic processes. This newly recognized role of the sweet taste receptor makes this receptor a potential novel therapeutic target for the treatment of obesity and related metabolic dysfunctions, such as diabetes and hyperlipidemia.
Figures

References
-
- Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav 2011; 105:4–13 - PubMed
-
- Pin JP, Kniazeff J, Goudet C, et al. The activation mechanism of class-C G-protein coupled receptors. Biol Cell 2004; 96:335–342 - PubMed
-
- Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science (New York, NY) 2003; 301:850–853 - PubMed
-
- Iwatsuki K, Uneyama H. Sense of taste in the gastrointestinal tract. J Pharmacol Sci 2012; 118:123–128 - PubMed
-
- Tomonari H, Miura H, Nakayama A, et al. Galpha-gustducin is extensively coexpressed with sweet and bitter taste receptors in both the soft palate and fungiform papillae but has a different functional significance. Chem Senses 2012; 37:241–251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

