Limited brain metabolism changes differentiate between the progression and clearance of rabies virus
- PMID: 24763072
- PMCID: PMC3998930
- DOI: 10.1371/journal.pone.0087180
Limited brain metabolism changes differentiate between the progression and clearance of rabies virus
Abstract
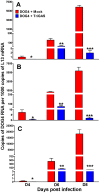
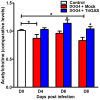
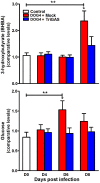
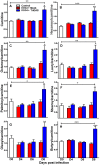
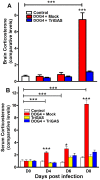
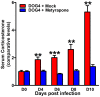
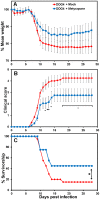
Central nervous system (CNS) metabolic profiles were examined from rabies virus (RABV)-infected mice that were either mock-treated or received post-exposure treatment (PET) with a single dose of the live recombinant RABV vaccine TriGAS. CNS tissue harvested from mock-treated mice at middle and late stage infection revealed numerous changes in energy metabolites, neurotransmitters and stress hormones that correlated with replication levels of viral RNA. Although the large majority of these metabolic changes were completely absent in the brains of TriGAS-treated mice most likely due to the strong reduction in virus spread, TriGAS treatment resulted in the up-regulation of the expression of carnitine and several acylcarnitines, suggesting that these compounds are neuroprotective. The most striking change seen in mock-treated RABV-infected mice was a dramatic increase in brain and serum corticosterone levels, with the later becoming elevated before clinical signs or loss of body weight occurred. We speculate that the rise in corticosterone is part of a strategy of RABV to block the induction of immune responses that would otherwise interfere with its spread. In support of this concept, we show that pharmacological intervention to inhibit corticosterone biosynthesis, in the absence of vaccine treatment, significantly reduces the pathogenicity of RABV. Our results suggest that widespread metabolic changes, including hypothalamic-pituitary-adrenal axis activation, contribute to the pathogenesis of RABV and that preventing these alterations early in infection with PET or pharmacological blockade helps protect brain homeostasis, thereby reducing disease mortality.
Conflict of interest statement
Figures
Similar articles
-
Postexposure treatment with the live-attenuated rabies virus (RV) vaccine TriGAS triggers the clearance of wild-type RV from the Central Nervous System (CNS) through the rapid induction of genes relevant to adaptive immunity in CNS tissues.J Virol. 2012 Mar;86(6):3200-10. doi: 10.1128/JVI.06699-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238315 Free PMC article.
-
Comprehensive Analysis of Protein Acetylation and Glucose Metabolism in Mouse Brains Infected with Rabies Virus.J Virol. 2022 Feb 23;96(4):e0194221. doi: 10.1128/JVI.01942-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878915 Free PMC article.
-
Intramuscular inoculation of mice with the live-attenuated recombinant rabies virus TriGAS results in a transient infection of the draining lymph nodes and a robust, long-lasting protective immune response against rabies.J Virol. 2013 Feb;87(3):1834-41. doi: 10.1128/JVI.02589-12. Epub 2012 Nov 28. J Virol. 2013. PMID: 23192867 Free PMC article.
-
The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms.Viruses. 2024 Nov 14;16(11):1774. doi: 10.3390/v16111774. Viruses. 2024. PMID: 39599888 Free PMC article. Review.
-
Role of chemokines in rabies pathogenesis and protection.Adv Virus Res. 2011;79:73-89. doi: 10.1016/B978-0-12-387040-7.00005-6. Adv Virus Res. 2011. PMID: 21601043 Free PMC article. Review.
Cited by
-
Quantitative Proteome Profiling of Street Rabies Virus-Infected Mouse Hippocampal Synaptosomes.Curr Microbiol. 2016 Sep;73(3):301-311. doi: 10.1007/s00284-016-1061-5. Epub 2016 May 7. Curr Microbiol. 2016. PMID: 27155843
-
Rabies virus infection is associated with variations in calbindin D-28K and calretinin mRNA expression levels in mouse brain tissue.Arch Virol. 2023 Apr 18;168(5):143. doi: 10.1007/s00705-023-05753-2. Arch Virol. 2023. PMID: 37069450 Free PMC article.
-
Rabies virus infection is associated with alterations in the expression of parvalbumin and secretagogin in mice brain.Metab Brain Dis. 2021 Aug;36(6):1267-1275. doi: 10.1007/s11011-021-00717-4. Epub 2021 Mar 30. Metab Brain Dis. 2021. PMID: 33783673 Free PMC article.
-
Subversion of the Immune Response by Rabies Virus.Viruses. 2016 Aug 19;8(8):231. doi: 10.3390/v8080231. Viruses. 2016. PMID: 27548204 Free PMC article. Review.
-
Alterations of Gut Microbiome and Metabolite Profiling in Mice Infected by Schistosoma japonicum.Front Immunol. 2020 Oct 8;11:569727. doi: 10.3389/fimmu.2020.569727. eCollection 2020. Front Immunol. 2020. PMID: 33162984 Free PMC article.
References
-
- WHO (2004) WHO technical report series 931. Geneva, Switzerland.
-
- Faber M, Dietzschold B, Li J (2009) Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health 56: 262–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

