Mammalian cells contain two functionally distinct PBAF complexes incorporating different isoforms of PHF10 signature subunit
- PMID: 24763304
- PMCID: PMC4111760
- DOI: 10.4161/cc.28922
Mammalian cells contain two functionally distinct PBAF complexes incorporating different isoforms of PHF10 signature subunit
Abstract
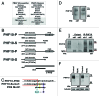
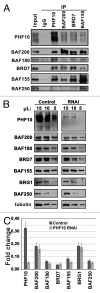
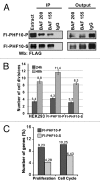
The PBAF subtype of the mammalian chromatin remodeling SWI/SNF complex has wide and diverse functions in transcription regulation and development, being both transcription activator and repressor. However, a mechanism accounting for such functional diversity remains unclear. Human PHF10/BAF45a subunit of the PBAF complex plays an important role in brain development but has not been studied sufficiently. We have shown that the PHF10 gene encodes 2 types of evolutionarily conserved, ubiquitously expressed isoforms that are incorporated into the PBAF complex in a mutually exclusive manner. One isoform contains C-terminal tandem PHD fingers, which in the other isoform are replaced by the consensus sequence for phosphorylation-dependent SUMO 1 conjugation (PDSM). PBAF complexes containing different PHF10 isoforms can bind to the promoters of the same genes but produce different effects on the recruitment of Pol II to the promoter and on the level of gene transcription. In addition, it is only the PBAF with PHD-containing isoform that activates proliferation. Our study demonstrates the existence of functionally different PBAF complexes in mammalian cell. It also provides an insight into the molecular structure and role of human PHF10/BAF45a and characterizes it as an essential PBAF subunit.
Keywords: PBAF signature subunits; PDSM motif; PHD domains; PHF10/BAF45; SWI/SNF chromatin remodeling complex; functionally distinct isoforms; sumoylation.
Figures



Similar articles
-
The sequential phosphorylation of PHF10 subunit of the PBAF chromatin-remodeling complex determines different properties of the PHF10 isoforms.Biol Open. 2020 Jan 15;9(1):bio043943. doi: 10.1242/bio.043943. Biol Open. 2020. PMID: 31911482 Free PMC article.
-
Stability of the PHF10 subunit of PBAF signature module is regulated by phosphorylation: role of β-TrCP.Sci Rep. 2017 Jul 17;7(1):5645. doi: 10.1038/s41598-017-05944-3. Sci Rep. 2017. PMID: 28717195 Free PMC article.
-
[PHF10 isoforms are phosphorylated in the PBAF mammalian chromatin remodeling complex].Mol Biol (Mosk). 2016 Mar-Apr;50(2):320-6. doi: 10.7868/S0026898416010031. Mol Biol (Mosk). 2016. PMID: 27239853 Russian.
-
Conserved Structure and Evolution of DPF Domain of PHF10-The Specific Subunit of PBAF Chromatin Remodeling Complex.Int J Mol Sci. 2021 Oct 15;22(20):11134. doi: 10.3390/ijms222011134. Int J Mol Sci. 2021. PMID: 34681795 Free PMC article. Review.
-
Polybromo-1: the chromatin targeting subunit of the PBAF complex.Biochimie. 2009 Mar;91(3):309-19. doi: 10.1016/j.biochi.2008.10.019. Epub 2008 Dec 3. Biochimie. 2009. PMID: 19084573 Free PMC article. Review.
Cited by
-
Chromatin Remodeling Complex PBAF Activates and Represses Inflammatory Genes.Dokl Biochem Biophys. 2023 Dec;513(1):332-336. doi: 10.1134/S1607672923700539. Epub 2023 Dec 8. Dokl Biochem Biophys. 2023. PMID: 38066320
-
Differential Impact of Random GC Tetrad Binding and Chromatin Events on Transcriptional Inhibition by Olivomycin A.Int J Mol Sci. 2022 Aug 9;23(16):8871. doi: 10.3390/ijms23168871. Int J Mol Sci. 2022. PMID: 36012127 Free PMC article.
-
PHD finger protein 10 promotes cell proliferation by regulating CD44 transcription in gastric cancer.Heliyon. 2024 Apr 3;10(7):e29109. doi: 10.1016/j.heliyon.2024.e29109. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38601625 Free PMC article.
-
A novel chromatin-remodeling complex variant, dcPBAF, is involved in maintaining transcription in differentiated neurons.Front Cell Dev Biol. 2023 Nov 14;11:1271598. doi: 10.3389/fcell.2023.1271598. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033872 Free PMC article.
-
Baf45a Mediated Chromatin Remodeling Promotes Transcriptional Activation for Osteogenesis and Odontogenesis.Front Endocrinol (Lausanne). 2022 Jan 3;12:763392. doi: 10.3389/fendo.2021.763392. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046892 Free PMC article.
References
-
- Moshkin YM, Chalkley GE, Kan TW, Reddy BA, Ozgur Z, van Ijcken WF, Dekkers DH, Demmers JA, Travers AA, Verrijzer CP. Remodelers organize cellular chromatin by counteracting intrinsic histone-DNA sequence preferences in a class-specific manner. Mol Cell Biol. 2012;32:675–88. doi: 10.1128/MCB.06365-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
