Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop
- PMID: 24763408
- PMCID: PMC3999033
- DOI: 10.1371/journal.pone.0095767
Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop
Abstract
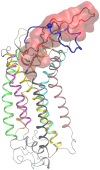
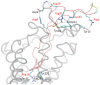
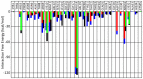
The binding of protein HIV-1 gp120 to coreceptors CCR5 or CXCR4 is a key step of the HIV-1 entry to the host cell, and is predominantly mediated through the V3 loop fragment of HIV-1 gp120. In the present work, we delineate the molecular recognition of chemokine receptor CCR5 by a dual tropic HIV-1 gp120 V3 loop, using a comprehensive set of computational tools predominantly based on molecular dynamics simulations and free energy calculations. We report, what is to our knowledge, the first complete HIV-1 gp120 V3 loop : CCR5 complex structure, which includes the whole V3 loop and the N-terminus of CCR5, and exhibits exceptional agreement with previous experimental findings. The computationally derived structure sheds light into the functional role of HIV-1 gp120 V3 loop and CCR5 residues associated with the HIV-1 coreceptor activity, and provides insights into the HIV-1 coreceptor selectivity and the blocking mechanism of HIV-1 gp120 by maraviroc. By comparing the binding of the specific dual tropic HIV-1 gp120 V3 loop with CCR5 and CXCR4, we observe that the HIV-1 gp120 V3 loop residues 13-21, which include the tip, share nearly identical structural and energetic properties in complex with both coreceptors. This result paves the way for the design of dual CCR5/CXCR4 targeted peptides as novel potential anti-AIDS therapeutics.
Conflict of interest statement
Figures
References
-
- Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, et al. (1997) HIV-1 tropism and co-receptor use. Nature 385: 495–496. - PubMed
-
- Weiss RA, Clapham PR (1996) Hot fusion of HIV. Nature 381: 647–648. - PubMed
-
- Chan DC, Kim PS (1998) HIV entry and its inhibition. Cell 93: 681–684. - PubMed
-
- Chan DC, Fass D, Berger JM, Kim PS (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89: 263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

