Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy
- PMID: 24763467
- PMCID: PMC4043633
- DOI: 10.1161/CIRCRESAHA.114.303772
Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy
Abstract
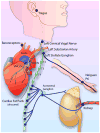
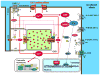
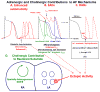
Autonomic nervous system activation can induce significant and heterogeneous changes of atrial electrophysiology and induce atrial tachyarrhythmias, including atrial tachycardia and atrial fibrillation (AF). The importance of the autonomic nervous system in atrial arrhythmogenesis is also supported by circadian variation in the incidence of symptomatic AF in humans. Methods that reduce autonomic innervation or outflow have been shown to reduce the incidence of spontaneous or induced atrial arrhythmias, suggesting that neuromodulation may be helpful in controlling AF. In this review, we focus on the relationship between the autonomic nervous system and the pathophysiology of AF and the potential benefit and limitations of neuromodulation in the management of this arrhythmia. We conclude that autonomic nerve activity plays an important role in the initiation and maintenance of AF, and modulating autonomic nerve function may contribute to AF control. Potential therapeutic applications include ganglionated plexus ablation, renal sympathetic denervation, cervical vagal nerve stimulation, baroreflex stimulation, cutaneous stimulation, novel drug approaches, and biological therapies. Although the role of the autonomic nervous system has long been recognized, new science and new technologies promise exciting prospects for the future.
Keywords: heart failure; myocardial infarction.
Figures





References
-
- Viskin S, Golovner M, Malov N, Fish R, Alroy I, Vila Y, Laniado S, Kaplinsky E, Roth A. Circadian variation of symptomatic paroxysmal atrial fibrillation. Data from almost 10 000 episodes. European Heart Journal. 1999;20:1429–1434. - PubMed
-
- Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG, Kus T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Autonomic neuroscience: basic & clinical. 2011;161:68–74. - PubMed
-
- Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

