Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management
- PMID: 24763468
- PMCID: PMC4043630
- DOI: 10.1161/CIRCRESAHA.114.302240
Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management
Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia in humans. The mechanisms that govern AF initiation and persistence are highly complex, of dynamic nature, and involve interactions across multiple temporal and spatial scales in the atria. This article aims to review the mathematical modeling and computer simulation approaches to understanding AF mechanisms and aiding in its management. Various atrial modeling approaches are presented, with descriptions of the methodological basis and advancements in both lower-dimensional and realistic geometry models. A review of the most significant mechanistic insights made by atrial simulations is provided. The article showcases the contributions that atrial modeling and simulation have made not only to our understanding of the pathophysiology of atrial arrhythmias, but also to the development of AF management approaches. A summary of the future developments envisioned for the field of atrial simulation and modeling is also presented. The review contends that computational models of the atria assembled with data from clinical imaging modalities that incorporate electrophysiological and structural remodeling could become a first line of screening for new AF therapies and approaches, new diagnostic developments, and new methods for arrhythmia prevention.
Keywords: arrhythmias, cardiac; atrial fibrillation; atrial remodeling; computer simulation.
Figures





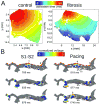
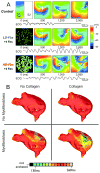

References
-
- Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Archives of internal medicine. 1995;155:469–473. - PubMed
-
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. - PubMed
-
- Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–2274. - PubMed
-
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circulation. Arrhythmia and electrophysiology. 2008;1:62–73. - PubMed
-
- Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiological reviews. 2011;91:265–325. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

