A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light
- PMID: 24763585
- PMCID: PMC4544835
- DOI: 10.1126/science.1251511
A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light
Abstract
In contrast to the wealth of catalytic systems that are available to control the stereochemistry of thermally promoted cycloadditions, few similarly effective methods exist for the stereocontrol of photochemical cycloadditions. A major unsolved challenge in the design of enantioselective catalytic photocycloaddition reactions has been the difficulty of controlling racemic background reactions that occur by direct photoexcitation of substrates while unbound to catalyst. Here, we describe a strategy for eliminating the racemic background reaction in asymmetric [2 + 2] photocycloadditions of α,β-unsaturated ketones to the corresponding cyclobutanes by using a dual-catalyst system consisting of a visible light-absorbing transition-metal photocatalyst and a stereocontrolling Lewis acid cocatalyst. The independence of these two catalysts enables broader scope, greater stereochemical flexibility, and better efficiency than previously reported methods for enantioselective photochemical cycloadditions.
Figures



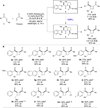
Comment in
-
Chemistry. A two-catalyst photochemistry route to homochiral rings.Science. 2014 Apr 25;344(6182):368-9. doi: 10.1126/science.1252965. Science. 2014. PMID: 24763578 No abstract available.
References
-
- Jacobsen EN, Pfaltz A, Yamamoto H. Comprehensive Asymmetric Catalysis. Berlin, New York: Springer; 1999.
-
- Ojima I. Catalytic Asymmetric Synthesis. 3rd ed. Hoboken, N.J.: John Wiley; 2010.
-
- Iriondo-Alberdi J, Greaney MF. Eur. J. Org. Chem. 2007;4801
-
- Hoffmann N. Chem. Rev. 2008;108:1052. - PubMed
-
- Rau H. Chem. Rev. 1983;83:535.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

