Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations
- PMID: 24764037
- PMCID: PMC4070270
- DOI: 10.1208/s12248-014-9599-2
Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations
Abstract
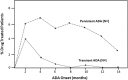
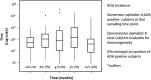
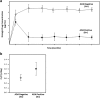
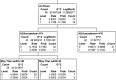
Immunogenicity is a significant concern for biologic drugs as it can affect both safety and efficacy. To date, the descriptions of product immunogenicity have varied not only due to different degrees of understanding of product immunogenicity at the time of licensing but also due to an evolving lexicon that has generated some confusion in the field. In recent years, there has been growing consensus regarding the data needed to assess product immunogenicity. Harmonization of the strategy for the elucidation of product immunogenicity by drug developers, as well as the use of defined common terminology, can benefit medical practitioners, health regulatory agencies, and ultimately the patients. Clearly, understanding the incidence, kinetics and magnitude of anti-drug antibody (ADA), its neutralizing ability, cross-reactivity with endogenous molecules or other marketed biologic drugs, and related clinical impact may enhance clinical management of patients treated with biologic drugs. To that end, the authors present terms and definitions for describing and analyzing clinical immunogenicity data and suggest approaches to data presentation, emphasizing associations of ADA development with pharmacokinetics, efficacy, and safety that are necessary to assess the clinical relevance of immunogenicity.
Figures


References
-
- Schellekens H, Casadevall N. Immunogenicity of recombinant human proteins: causes and consequences. J Neurol. 2004;251(Suppl 2):II4–II9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

