Evaluation of the HC-04 cell line as an in vitro model for mechanistic assessment of changes in hepatic cytochrome P450 3A during adenovirus infection
- PMID: 24764148
- PMCID: PMC4053995
- DOI: 10.1124/dmd.113.056663
Evaluation of the HC-04 cell line as an in vitro model for mechanistic assessment of changes in hepatic cytochrome P450 3A during adenovirus infection
Abstract
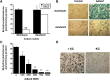
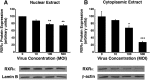
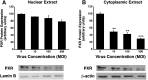
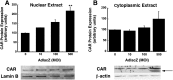
HC-04 cells were evaluated as an in vitro model for mechanistic study of changes in the function of hepatic CYP3A during virus infection. Similar to in vivo observations, infection with a first generation recombinant adenovirus significantly inhibited CYP3A4 catalytic activity in an isoform-specific manner. Virus (MOI 100) significantly reduced expression of the retinoid X receptor (RXR) by 30% 96 hours after infection. Cytoplasmic concentrations of the pregnane X receptor (PXR) were reduced by 50%, whereas the amount of the constitutive androstane receptor (CAR) in the nuclear fraction doubled with respect to uninfected controls. Hepatocyte nuclear factor 4α (HNF-4α) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) were also reduced by ∼70% during infection. Virus suppressed CYP3A4 activity in the presence of the PXR agonist rifampicin and did not affect CYP3A4 activity in the presence of the CAR agonist CITCO [6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime], suggesting that virus-induced modification of PXR may be responsible for observed changes in hepatic CYP3A4. The HC-04 cell line is easy to maintain, and CYP3A4 in these cells was responsive to known inducers and suppressors. Dexamethasone (200 μM) and phenobarbital (500 μM) increased activity by 230 and 124%, whereas ketoconazole (10 μM) and lipopolysaccharide (LPS) (10 μg/ml) reduced activity by 90 and 92%, respectively. This suggests that HC-04 cells can be a valuable tool for mechanistic study of drug metabolism during infection and for routine toxicological screening of novel compounds prior to use in the clinic.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
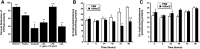





Similar articles
-
Induction of human CYP2A6 is mediated by the pregnane X receptor with peroxisome proliferator-activated receptor-gamma coactivator 1alpha.J Pharmacol Exp Ther. 2006 Nov;319(2):693-702. doi: 10.1124/jpet.106.107573. Epub 2006 Jul 20. J Pharmacol Exp Ther. 2006. PMID: 16857725
-
Dual roles of nuclear receptor liver X receptor α (LXRα) in the CYP3A4 expression in human hepatocytes as a positive and negative regulator.Biochem Pharmacol. 2013 Aug 1;86(3):428-36. doi: 10.1016/j.bcp.2013.05.016. Epub 2013 May 31. Biochem Pharmacol. 2013. PMID: 23732298
-
Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression.Drug Metab Dispos. 2006 May;34(5):756-64. doi: 10.1124/dmd.105.007575. Epub 2006 Feb 2. Drug Metab Dispos. 2006. PMID: 16455805 Free PMC article.
-
Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha.Mol Pharmacol. 2005 Sep;68(3):747-57. doi: 10.1124/mol.105.013169. Epub 2005 Jun 2. Mol Pharmacol. 2005. PMID: 15933212
-
Insights into CYP2B6-mediated drug-drug interactions.Acta Pharm Sin B. 2016 Sep;6(5):413-425. doi: 10.1016/j.apsb.2016.07.016. Epub 2016 Aug 9. Acta Pharm Sin B. 2016. PMID: 27709010 Free PMC article. Review.
Cited by
-
Immunization and Drug Metabolizing Enzymes: Focus on Hepatic Cytochrome P450 3A.Expert Rev Vaccines. 2021 May;20(5):623-634. doi: 10.1080/14760584.2021.1899818. Epub 2021 Mar 18. Expert Rev Vaccines. 2021. PMID: 33666138 Free PMC article.
-
Integrin Receptors Play a Key Role in the Regulation of Hepatic CYP3A.Drug Metab Dispos. 2016 May;44(5):758-70. doi: 10.1124/dmd.115.068874. Epub 2016 Feb 11. Drug Metab Dispos. 2016. PMID: 26868618 Free PMC article.
-
The Role of CYP3A in Health and Disease.Biomedicines. 2022 Oct 24;10(11):2686. doi: 10.3390/biomedicines10112686. Biomedicines. 2022. PMID: 36359206 Free PMC article. Review.
-
The Role of Cytochrome P450 Enzymes in COVID-19 Pathogenesis and Therapy.Front Pharmacol. 2022 Feb 2;13:791922. doi: 10.3389/fphar.2022.791922. eCollection 2022. Front Pharmacol. 2022. PMID: 35185562 Free PMC article. Review.
-
Assessment of In Vitro Models of the Human Buccal Mucosa for Vaccine and Adjuvant Development.Mol Pharm. 2025 Jun 2;22(6):2868-2880. doi: 10.1021/acs.molpharmaceut.4c01186. Epub 2025 Mar 26. Mol Pharm. 2025. PMID: 40139941
References
-
- Andersson TB, Kanebratt KP, Kenna JG. (2012) The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol 8:909–920 - PubMed
-
- Boudjelal M, Wang Z, Voorhees JJ, Fisher GJ. (2000) Ubiquitin/proteasome pathway regulates levels of retinoic acid receptor gamma and retinoid X receptor alpha in human keratinocytes. Cancer Res 60:2247–2252 - PubMed
-
- Brtko J, Dvorak Z. (2011) Role of retinoids, rexinoids and thyroid hormone in the expression of cytochrome p450 enzymes. Curr Drug Metab 12:71–88 - PubMed
-
- Callahan SM (2005a) Recombinant Adenoviral-Meditated Alterations of Cytochrome P450 3A2 and 2C11 Ph.D. thesis. The University of Texas at Austin, Austin, TX.
-
- Callahan SM, Ming X, Lu SK, Brunner LJ, Croyle MA. (2005b) Considerations for use of recombinant adenoviral vectors: dose effect on hepatic cytochromes P450. J Pharmacol Exp Ther 312:492–501 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

