Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice
- PMID: 24764191
- PMCID: PMC4036470
- DOI: 10.1242/dmm.015750
Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice
Abstract
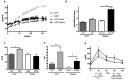
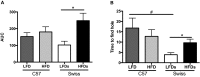
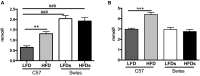
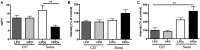
One of the tissues or organs affected by diabetes is the nervous system, predominantly the peripheral system (peripheral polyneuropathy and/or painful peripheral neuropathy) but also the central system with impaired learning, memory and mental flexibility. The aim of this study was to test the hypothesis that the pre-diabetic or diabetic condition caused by a high-fat diet (HFD) can damage both the peripheral and central nervous systems. Groups of C57BL6 and Swiss Webster mice were fed a diet containing 60% fat for 8 months and compared to control and streptozotocin (STZ)-induced diabetic groups that were fed a standard diet containing 10% fat. Aspects of peripheral nerve function (conduction velocity, thermal sensitivity) and central nervous system function (learning ability, memory) were measured at assorted times during the study. Both strains of mice on HFD developed impaired glucose tolerance, indicative of insulin resistance, but only the C57BL6 mice showed statistically significant hyperglycemia. STZ-diabetic C57BL6 mice developed learning deficits in the Barnes maze after 8 weeks of diabetes, whereas neither C57BL6 nor Swiss Webster mice fed a HFD showed signs of defects at that time point. By 6 months on HFD, Swiss Webster mice developed learning and memory deficits in the Barnes maze test, whereas their peripheral nervous system remained normal. In contrast, C57BL6 mice fed the HFD developed peripheral nerve dysfunction, as indicated by nerve conduction slowing and thermal hyperalgesia, but showed normal learning and memory functions. Our data indicate that STZ-induced diabetes or a HFD can damage both peripheral and central nervous systems, but learning deficits develop more rapidly in insulin-deficient than in insulin-resistant conditions and only in Swiss Webster mice. In addition to insulin impairment, dyslipidemia or adiponectinemia might determine the neuropathy phenotype.
Keywords: Glucose; High-fat diet; Insulin; Neuropathy.
© 2014. Published by The Company of Biologists Ltd.
Figures


References
-
- Ahima R. S. (2006). Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity (Silver Spring) 14 Suppl. 1, 9S–15S - PubMed
-
- Ansquer J. C., Foucher C., Aubonnet P., Le Malicot K. (2009). Fibrates and microvascular complications in diabetes – insight from the FIELD study. Curr. Pharm. Des. 15, 537–552 - PubMed
-
- Baron R., Tölle T. R., Gockel U., Brosz M., Freynhagen R. (2009). A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain 146, 34–40 - PubMed
-
- Biessels G. J., Deary I. J., Ryan C. M. (2008). Cognition and diabetes: a lifespan perspective. Lancet Neurol. 7, 184–190 - PubMed
-
- Calcutt N. A. (2004). Modeling diabetic sensory neuropathy in rats. Methods Mol. Med. 99, 55–65 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

