Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)--10-year follow-up of patients who previously participated in an MPS VI Survey Study
- PMID: 24764221
- PMCID: PMC4107078
- DOI: 10.1002/ajmg.a.36584
Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)--10-year follow-up of patients who previously participated in an MPS VI Survey Study
Abstract
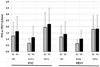
Mucopolysaccharidosis VI (MPS VI) is a clinically heterogeneous and progressive disorder with multiorgan manifestations caused by deficient N-acetylgalactosamine-4-sulfatase activity. A cross-sectional Survey Study in individuals (n = 121) affected with MPS VI was conducted between 2001 and 2002 to establish demographics, urinary glycosaminoglycan (GAG) levels, and clinical progression of disease. We conducted a Resurvey Study (ClinicalTrials.gov: NCT01387854) to obtain 10-year follow-up data, including medical histories and clinical assessments (n = 59), and survival status over 12 years (n = 117). Patients received a mean (SD) of 6.8 (2.2) years of galsulfase ERT between baseline (Survey Study) and follow-up. ERT patients increased in height by 20.4 cm in the 4-7-year-old baseline age group and by 16.8 cm in the 8-12-year-old baseline age group. ERT patients <13 years-old demonstrated improvement in forced vital capacity (FVC) by 68% and forced expiratory volume in 1 sec (FEV1) by 55%, and those ≥13 years-old increased FVC by 12.8% and maintained FEV1. Patients with >200 µg/mg baseline uGAG levels increased FVC by 48% in the <13-year-old baseline age group and by 15% in the ≥13-year-old baseline age group. ERT patients who completed the 6-min walk test demonstrated a mean (SD) increase of 65.7 (100.6) m. Cardiac outcomes did not significantly improve or worsen. Observed mortality rate among naïve patients was 50% (7/14) and 16.5% (17/103) in the ERT group (unadjusted hazard ratio, 0.24; 95% CI, 0.10-0.59). Long-term galsulfase ERT was associated with improvements in pulmonary function and endurance, stabilized cardiac function and increased survival.
Keywords: Maroteaux-Lamy syndrome; N-acetylgalactosamine-4-sulfatase; enzyme replacement therapy; exercise tolerance; follow-up studies; mucopolysaccharidosis VI; multicenter study [publication type]; respiratory function tests; survival rate.
© 2014 Wiley Periodicals, Inc.
Figures



References
-
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. [no authors] - PubMed
-
- Azevedo AC, Schwartz IV, Kalakun L, Brustolin S, Burin MG, Beheregaray AP, Leistner S, Giugliani C, Rosa M, Barrios P, Marinho D, Esteves P, Valadares E, Boy R, Horovitz D, Mabe P, da Silva LC, de Souza IC, Ribeiro M, Martins AM, Palhares D, Kim CA, Giugliani R. Clinical and biochemical study of 28 patients with mucopolysaccharidosis type VI. Clin Genet. 2004;66(3):208–213. - PubMed
-
- BioMarin. Naglazyme (galsulfase) Prescribing Information. 2013
-
- Brands M, Oussoren E, Ruijter G, Vollebregt A, van den Hout H, Joosten K, Hop W, Plug I, van der Ploeg A. Up to five years experience with 11 mucopolysaccharidosis type VI patients. Mol Genet Metab. 2013b;109(1):70–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

