Vipp1 is essential for the biogenesis of Photosystem I but not thylakoid membranes in Synechococcus sp. PCC 7002
- PMID: 24764304
- PMCID: PMC4047364
- DOI: 10.1074/jbc.M114.555631
Vipp1 is essential for the biogenesis of Photosystem I but not thylakoid membranes in Synechococcus sp. PCC 7002
Abstract
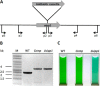
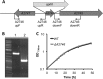
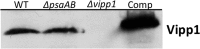
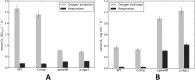
The biogenesis of thylakoid membranes in cyanobacteria is presently not well understood, but the vipp1 gene product has been suggested to play an important role in this process. Previous studies in Synechocystis sp. PCC 6803 reported that vipp1 (sll0617) was essential. By constructing a fully segregated null mutant in vipp1 (SynPCC7002_A0294) in Synechococcus sp. PCC 7002, we show that Vipp1 is not essential. Spectroscopic studies revealed that Photosystem I (PS I) was below detection limits in the vipp1 mutant, but Photosystem II (PS II) was still assembled and was active. Thylakoid membranes were still observed in vipp1 mutant cells and resembled those in a psaAB mutant that completely lacks PS I. When the vipp1 mutation was complemented with the orthologous vipp1 gene from Synechocystis sp. PCC 6803 that was expressed from the strong P(cpcBA) promoter, PS I content and activities were restored to normal levels, and cells again produced thylakoids that were indistinguishable from those of wild type. Transcription profiling showed that psaAB transcripts were lower in abundance in the vipp1 mutant. However, when the yfp gene was expressed from the P(psaAB) promoter in the presence and the absence of Vipp1, no difference in YFP expression was observed, which shows that Vipp1 is not a transcription factor for the psaAB genes. This study shows that thylakoids are still produced in the absence of Vipp1 and that normal thylakoid biogenesis in Synechococcus sp. PCC 7002 requires expression and biogenesis of PS I, which in turn requires Vipp1.
Keywords: Cyanobacteria; Electron Microscopy (EM); Membrane Biogenesis; Photosynthesis; Photosystem I; Protein Translocation.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


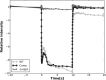


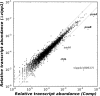

Similar articles
-
Depletion of Vipp1 in Synechocystis sp. PCC 6803 affects photosynthetic activity before the loss of thylakoid membranes.FEMS Microbiol Lett. 2009 Mar;292(1):63-70. doi: 10.1111/j.1574-6968.2008.01470.x. FEMS Microbiol Lett. 2009. PMID: 19222583
-
Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis?Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4243-8. doi: 10.1073/pnas.061501198. Proc Natl Acad Sci U S A. 2001. PMID: 11274448 Free PMC article.
-
Assembly of photosystem I. I. Inactivation of the rubA gene encoding a membrane-associated rubredoxin in the cyanobacterium Synechococcus sp. PCC 7002 causes a loss of photosystem I activity.J Biol Chem. 2002 Jun 7;277(23):20343-54. doi: 10.1074/jbc.M201103200. Epub 2002 Mar 25. J Biol Chem. 2002. PMID: 11914373
-
Possible function of VIPP1 in maintaining chloroplast membranes.Biochim Biophys Acta. 2015 Sep;1847(9):831-7. doi: 10.1016/j.bbabio.2015.02.013. Epub 2015 Feb 25. Biochim Biophys Acta. 2015. PMID: 25725437 Review.
-
Vipp1: a very important protein in plastids?!J Exp Bot. 2012 Feb;63(4):1699-712. doi: 10.1093/jxb/err357. Epub 2011 Nov 29. J Exp Bot. 2012. PMID: 22131161 Review.
Cited by
-
Structural basis for Vipp1 membrane binding: from loose coats and carpets to ring and rod assemblies.Nat Struct Mol Biol. 2025 Mar;32(3):555-570. doi: 10.1038/s41594-024-01399-z. Epub 2024 Oct 8. Nat Struct Mol Biol. 2025. PMID: 39379528 Free PMC article.
-
Mechanism for Vipp1 spiral formation, ring biogenesis, and membrane repair.Nat Struct Mol Biol. 2025 Mar;32(3):571-584. doi: 10.1038/s41594-024-01401-8. Epub 2024 Nov 11. Nat Struct Mol Biol. 2025. PMID: 39528797 Free PMC article.
-
Membrane remodelling in bacteria.J Struct Biol. 2016 Oct;196(1):3-14. doi: 10.1016/j.jsb.2016.05.010. Epub 2016 Jun 2. J Struct Biol. 2016. PMID: 27265614 Free PMC article. Review.
-
Structure, biogenesis, and evolution of thylakoid membranes.Plant Cell. 2024 Oct 3;36(10):4014-4035. doi: 10.1093/plcell/koae102. Plant Cell. 2024. PMID: 38567528 Free PMC article. Review.
-
Absolute quantification of cellular levels of photosynthesis-related proteins in Synechocystis sp. PCC 6803.Photosynth Res. 2023 Mar;155(3):219-245. doi: 10.1007/s11120-022-00990-z. Epub 2022 Dec 21. Photosynth Res. 2023. PMID: 36542271 Free PMC article.
References
-
- De Marais D. J. (2000) When did photosynthesis emerge on Earth? Science 289, 1703–1705 - PubMed
-
- Jordan P., Fromme P., Witt H. T., Klukas O., Saenger W., Krauss N. (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 909–917 - PubMed
-
- Vothknecht U. C., Westhoff P. (2001) Biogenesis and origin of thylakoid membranes. Biochim. Biophys. Acta 1541, 91–101 - PubMed
-
- Li H.-M., Kaneko Y., Keegstra K. (1994) Molecular cloning of a chloroplastic proteinassociated with both the envelope and thylakoid membranes. Plant Mol. Biol. 25, 619–632 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

