Requirement for CDK6 in MLL-rearranged acute myeloid leukemia
- PMID: 24764564
- PMCID: PMC4190617
- DOI: 10.1182/blood-2014-02-558114
Requirement for CDK6 in MLL-rearranged acute myeloid leukemia
Abstract
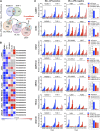
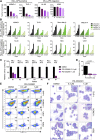
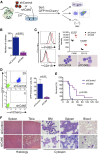
Chromosomal rearrangements involving the H3K4 methyltransferase mixed-lineage leukemia (MLL) trigger aberrant gene expression in hematopoietic progenitors and give rise to an aggressive subtype of acute myeloid leukemia (AML). Insights into MLL fusion-mediated leukemogenesis have not yet translated into better therapies because MLL is difficult to target directly, and the identity of the genes downstream of MLL whose altered transcription mediates leukemic transformation are poorly annotated. We used a functional genetic approach to uncover that AML cells driven by MLL-AF9 are exceptionally reliant on the cell-cycle regulator CDK6, but not its functional homolog CDK4, and that the preferential growth inhibition induced by CDK6 depletion is mediated through enhanced myeloid differentiation. CDK6 essentiality is also evident in AML cells harboring alternate MLL fusions and a mouse model of MLL-AF9-driven leukemia and can be ascribed to transcriptional activation of CDK6 by mutant MLL. Importantly, the context-dependent effects of lowering CDK6 expression are closely phenocopied by a small-molecule CDK6 inhibitor currently in clinical development. These data identify CDK6 as critical effector of MLL fusions in leukemogenesis that might be targeted to overcome the differentiation block associated with MLL-rearranged AML, and underscore that cell-cycle regulators may have distinct, noncanonical, and nonredundant functions in different contexts.
© 2014 by The American Society of Hematology.
Figures



Comment in
-
CDK6, a new target in MLL-driven leukemia.Blood. 2014 Jul 3;124(1):5-6. doi: 10.1182/blood-2014-05-572917. Blood. 2014. PMID: 24993876
References
-
- Döhner H, Estey EH, Amadori S, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. - PubMed
-
- Grimwade D, Hills RK, Moorman AV, et al. National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. - PubMed
-
- Krauter J, Wagner K, Schäfer I, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27(18):3000–3006. - PubMed
-
- Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

