Are patients with Parkinson's disease blind to blindsight?
- PMID: 24764573
- PMCID: PMC4032103
- DOI: 10.1093/brain/awu094
Are patients with Parkinson's disease blind to blindsight?
Abstract
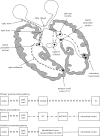
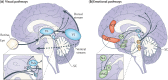
In Parkinson's disease, visual dysfunction is prominent. Visual hallucinations can be a major hallmark of late stage disease, but numerous visual deficits also occur in early stage Parkinson's disease. Specific retinopathy, deficits in the primary visual pathway and the secondary ventral and dorsal pathways, as well as dysfunction of the attention pathways have all been posited as causes of hallucinations in Parkinson's disease. We present data from patients with Parkinson's disease that contrast with a known neuro-ophthalmological syndrome, termed 'blindsight'. In this syndrome, there is an absence of conscious object identification, but preserved 'guess' of the location of a stimulus, preserved reflexive saccades and motion perception and preserved autonomical and expressive reactions to negative emotional facial expressions. We propose that patients with Parkinson's disease have the converse of blindsight, being 'blind to blindsight'. As such they preserve conscious vision, but show erroneous 'guess' localization of visual stimuli, poor saccades and motion perception, and poor emotional face perception with blunted autonomic reaction. Although a large data set on these deficits in Parkinson's disease has been accumulated, consolidation into one specific syndrome has not been proposed. Focusing on neuropathological and physiological data from two phylogenetically old and subconscious pathways, the retino-colliculo-thalamo-amygdala and the retino-geniculo-extrastriate pathways, we propose that aberrant function of these systems, including pathologically inhibited superior colliculus activity, deficient corollary discharges to the frontal eye fields, dysfunctional pulvinar, claustrum and amygdaloid subnuclei of the amygdala, the latter progressively burdened with Lewy bodies, underlie this syndrome. These network impairments are further corroborated by the concept of the 'silent amygdala'. Functionally being 'blind to blindsight' may facilitate the highly distinctive 'presence' or 'passage' hallucinations of Parkinson's disease and can help to explain handicaps in driving capacities and dysfunctional 'theory of mind'. We propose this synthesis to prompt refined neuropathological and neuroimaging studies on the pivotal nuclei in these pathways in order to better understand the networks underpinning this newly conceptualized syndrome in Parkinson's disease.
Keywords: Parkinson’s disease; blindsight; hallucinations; pre-emptive perception; superior colliculus.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. - PubMed
-
- Allen CP, Sumner P, Chambers CD. The Timing and Neuroanatomy of Conscious Vision as Revealed by TMS-induced Blindsight. J Cogn Neurosci. 2014 Advance Access published on January 6, 2014, doi:10.1162/jocn_a_00557. - PubMed
-
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson's disease. Brain. 2009;132:1128–45. - PubMed
-
- Archibald NK, Hutton SB, Clarke MP, Mosimann UP, Burn DJ. Visual exploration in Parkinson's disease and Parkinson's disease dementia. Brain. 2013;136:739–50. - PubMed
-
- Assogna F, Pontieri FE, Caltagirone C, Spalletta G. The recognition of facial emotion expressions in Parkinson's disease. Eur Neuropsychopharmacol. 2008;18:835–48. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

