Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice
- PMID: 24764578
- PMCID: PMC4001125
- DOI: 10.1158/2326-6066.CIR-13-0145
Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice
Abstract
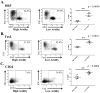
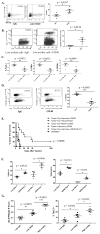
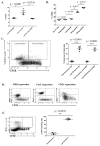
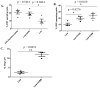
A major barrier to vaccines in cancer treatment is their failure to activate and maintain a complete cancer-specific CD8(+) effector T-cell repertoire. Low-avidity T cells are more likely to escape clonal deletion in the thymus when compared with high-avidity T cells, and therefore comprise the major population of effector T cells available for activation in patients with cancer. However, low-avidity T cells fail to traffic into the tumor microenvironment and function in eradicating tumor under optimal vaccination conditions as opposed to high-avidity T cells that escape clonal deletion and function in tumor killing. We used high- and low-avidity T-cell receptor transgenic CD8(+) T cells specific for the immunodominant epitope HER2/neu (RNEU420-429) to identify signaling pathways responsible for the inferior activity of the low-avidity T cells. Adoptive transfer of these cells into tumor-bearing vaccinated mice identified the members of apoptosis pathways that are upregulated in low-avidity T cells. The increased expression of proapoptotic proteins by low-avidity T cells promoted their own cell death and also that of other tumor-specific CD8(+) T cells within their local environment. Importantly, we show that this proapoptotic effect can be overcome by using a strong costimulatory signal that prevents the activation-induced cell death and enables the low-avidity T cells to traffic into the tumor and assist in tumor clearance. These findings identify new therapeutic opportunities for activating the most potent anticancer T-cell responses.
Conflict of interest statement
Figures


Similar articles
-
Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells.PLoS One. 2012;7(2):e31962. doi: 10.1371/journal.pone.0031962. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359647 Free PMC article.
-
Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice.Breast Cancer Res. 2012 Mar 7;14(2):R39. doi: 10.1186/bcr3135. Breast Cancer Res. 2012. PMID: 22397502 Free PMC article.
-
CD4(+) T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8(+) T-cell Targeting of HER2/neu-Expressing Cancers.Cancer Immunol Res. 2015 May;3(5):455-63. doi: 10.1158/2326-6066.CIR-14-0208. Epub 2015 Mar 19. Cancer Immunol Res. 2015. PMID: 25791067 Free PMC article.
-
Mechanisms of tumor escape: role of tumor microenvironment in inducing apoptosis of cytolytic effector cells.Arch Immunol Ther Exp (Warsz). 2006 Sep-Oct;54(5):323-33. doi: 10.1007/s00005-006-0038-7. Epub 2006 Oct 6. Arch Immunol Ther Exp (Warsz). 2006. PMID: 17031467 Review.
-
High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors.Immunol Res. 2005;31(1):13-24. doi: 10.1385/IR:31:1:13. Immunol Res. 2005. PMID: 15591619 Review.
Cited by
-
A Chlamydia-Specific TCR-Transgenic Mouse Demonstrates Th1 Polyfunctionality with Enhanced Effector Function.J Immunol. 2017 Oct 15;199(8):2845-2854. doi: 10.4049/jimmunol.1700914. Epub 2017 Aug 30. J Immunol. 2017. PMID: 28855311 Free PMC article.
-
A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice.Cancer Immunol Res. 2017 Jun;5(6):468-479. doi: 10.1158/2326-6066.CIR-16-0284. Epub 2017 May 8. Cancer Immunol Res. 2017. PMID: 28483787 Free PMC article.
-
A CD40 Agonist and PD-1 Antagonist Antibody Reprogram the Microenvironment of Nonimmunogenic Tumors to Allow T-cell-Mediated Anticancer Activity.Cancer Immunol Res. 2019 Mar;7(3):428-442. doi: 10.1158/2326-6066.CIR-18-0061. Epub 2019 Jan 14. Cancer Immunol Res. 2019. PMID: 30642833 Free PMC article.
-
Biological and clinical significance of tumour-infiltrating lymphocytes in the era of immunotherapy: a multidimensional approach.Nat Rev Clin Oncol. 2025 Mar;22(3):163-181. doi: 10.1038/s41571-024-00984-x. Epub 2025 Jan 16. Nat Rev Clin Oncol. 2025. PMID: 39820025 Review.
-
Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer.JCI Insight. 2018 Oct 18;3(20):e122857. doi: 10.1172/jci.insight.122857. JCI Insight. 2018. PMID: 30333318 Free PMC article.
References
-
- Durrant LG, Spendlove I. Cancer vaccines entering Phase III clinical trials. Expert opinion on emerging drugs. 2003;8:489–500. - PubMed
-
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40. - PubMed
-
- Kawai K, Ohashi PS. Immunological function of a defined T-cell population tolerized to low-affinity self antigens. Nature. 1995;374:68–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

