PD-L1 expression in triple-negative breast cancer
- PMID: 24764583
- PMCID: PMC4000553
- DOI: 10.1158/2326-6066.CIR-13-0127
PD-L1 expression in triple-negative breast cancer
Abstract
Early-phase trials targeting the T-cell inhibitory molecule programmed cell death ligand 1 (PD-L1) have shown clinical efficacy in cancer. This study was undertaken to determine whether PD-L1 is overexpressed in triple-negative breast cancer (TNBC) and to investigate the loss of PTEN as a mechanism of PD-L1 regulation. The Cancer Genome Atlas (TCGA) RNA sequencing data showed significantly greater expression of the PD-L1 gene in TNBC (n = 120) compared with non-TNBC (n = 716; P < 0.001). Breast tumor tissue microarrays were evaluated for PD-L1 expression, which was present in 19% (20 of 105) of TNBC specimens. PD-L1(+) tumors had greater CD8(+) T-cell infiltrate than PD-L1(-) tumors (688 cells/mm vs. 263 cells/mm; P < 0.0001). To determine the effect of PTEN loss on PD-L1 expression, stable cell lines were generated using PTEN short hairpin RNA (shRNA). PTEN knockdown led to significantly higher cell-surface PD-L1 expression and PD-L1 transcripts, suggesting transcriptional regulation. Moreover, phosphoinositide 3-kinase (PI3K) pathway inhibition using the AKT inhibitor MK-2206 or rapamycin resulted in decreased PD-L1 expression, further linking PTEN and PI3K signaling to PD-L1 regulation. Coculture experiments were performed to determine the functional effect of altered PD-L1 expression. Increased PD-L1 cell surface expression by tumor cells induced by PTEN loss led to decreased T-cell proliferation and increased apoptosis. PD-L1 is expressed in 20% of TNBCs, suggesting PD-L1 as a therapeutic target in TNBCs. Because PTEN loss is one mechanism regulating PD-L1 expression, agents targeting the PI3K pathway may increase the antitumor adaptive immune responses.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
The authors report no potential conflicts of interest.
Figures
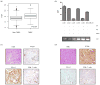
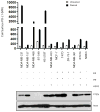
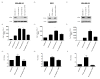


References
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. - PubMed
-
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. - PubMed
-
- Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

