The road ahead for cervical cancer prevention and control
- PMID: 24764711
- PMCID: PMC3997459
- DOI: 10.3747/co.21.1720
The road ahead for cervical cancer prevention and control
Abstract
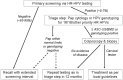
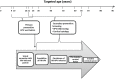
Since the early 1950s, Papanicolaou ("Pap") cytology screening has dramatically reduced cervical cancer mortality in most high-income settings. Currently, human papillomavirus (hpv) vaccination has the greatest potential to reduce the global burden of cervical cancer and precancerous lesions. However, as the prevalence of precancerous lesions declines, maintaining cytology as the primary screening test in settings with established programs might become less efficient. A reduction in test performance (sensitivity, specificity, and positive predictive value) would lead to an increase in unnecessary colposcopy referrals. Fortunately, hpv dna testing has emerged as a suitable candidate to replace cytology. Compared with the Pap test, hpv testing is less specific but much more sensitive in detecting high-grade precancerous lesions, less prone to human error, and more reproducible across settings. Linkage of hpv vaccination and screening registries could serve the added role of monitoring vaccine efficacy. As a triage test, cytology is expected to perform with sufficient accuracy because most hpv-positive smears would contain relevant abnormalities. This approach and others-for example, hpv testing followed by genotyping-are being evaluated in large population studies and have already been recommended in some settings. Other specific biomarkers that might perform well for screening and triage include hpv E6/E7 messenger rna testing, methylation of host or viral genes, and p16(INK4a) staining. Considering the rapid pace of major discoveries and the anticipated arrival of a nonavalent hpv vaccine (currently in phase iii trials), the evidence base in this field has become an elusive target and will continue to be an obstacle for policymakers.
Keywords: Cervical cancer; human papillomavirus; screening; vaccination.
Figures
Similar articles
-
Promising strategies for cervical cancer screening in the post-human papillomavirus vaccination era.Sex Health. 2010 Sep;7(3):376-82. doi: 10.1071/SH10022. Sex Health. 2010. PMID: 20719230 Review.
-
[Health technology assessment report: HPV DNA based primary screening for cervical cancer precursors].Epidemiol Prev. 2012 May-Aug;36(3-4 Suppl 1):e1-72. Epidemiol Prev. 2012. PMID: 22828243 Italian.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral.JAMA. 2002 Oct 9;288(14):1749-57. doi: 10.1001/jama.288.14.1749. JAMA. 2002. PMID: 12365959
-
P16/Ki-67 Dual Staining in Positive Human Papillomavirus DNA Testing for Predictive Diagnosis of Abnormal Cervical Lesions in Northeastern Thai Women.Asian Pac J Cancer Prev. 2022 Oct 1;23(10):3405-3411. doi: 10.31557/APJCP.2022.23.10.3405. Asian Pac J Cancer Prev. 2022. PMID: 36308365 Free PMC article.
Cited by
-
Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria.BMC Infect Dis. 2016 Apr 16;16:153. doi: 10.1186/s12879-016-1483-5. BMC Infect Dis. 2016. PMID: 27084683 Free PMC article.
-
HPV Testing from Dried Urine Spots as a Tool for Cervical Cancer Screening in Low-Income Countries.Biomed Res Int. 2015;2015:283036. doi: 10.1155/2015/283036. Epub 2015 Jun 9. Biomed Res Int. 2015. PMID: 26180790 Free PMC article. Clinical Trial.
-
What benefits and harms are important for a decision about cervical screening? A study of the perspective of different subgroups of women.Patient Prefer Adherence. 2019 Jul 1;13:1005-1017. doi: 10.2147/PPA.S193522. eCollection 2019. Patient Prefer Adherence. 2019. PMID: 31303748 Free PMC article.
-
HPV-FASTER: broadening the scope for prevention of HPV-related cancer.Nat Rev Clin Oncol. 2016 Feb;13(2):119-32. doi: 10.1038/nrclinonc.2015.146. Epub 2015 Sep 1. Nat Rev Clin Oncol. 2016. PMID: 26323382 Review.
-
Impact of knowledge and attitude on the utilization rate of cervical cancer screening tests among Ethiopian women: A systematic review and meta-analysis.PLoS One. 2020 Dec 8;15(12):e0239927. doi: 10.1371/journal.pone.0239927. eCollection 2020. PLoS One. 2020. PMID: 33290426 Free PMC article.
References
-
- Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193–206. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

