Challenges and outcomes of a randomized study of early nutrition support during autologous stem-cell transplantation
- PMID: 24764716
- PMCID: PMC3997464
- DOI: 10.3747/co.21.1820
Challenges and outcomes of a randomized study of early nutrition support during autologous stem-cell transplantation
Abstract
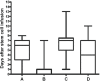
Patients undergoing myeloablative conditioning regimens and autologous stem-cell transplantation (asct) are at high risk of malnutrition. This randomized study aimed to determine if early nutrition support (commenced when oral intake is less than 80% of estimated requirements) compared with usual care (commenced when oral intake is less than 50% of estimated requirements) reduces weight loss in well-nourished patients undergoing high-nutritional-risk conditioning chemotherapy and asct. In the 50 well-nourished patients who were randomized, the outcomes evaluated included changes in weight and lean body mass (mid-upper arm circumference), length of stay, time to hemopoietic engraftment, and quality of life (Memorial Symptom Assessment Scale - Short Form). On secondary analysis, after exclusion of a single extreme outlier, both groups demonstrated significant weight loss over time (p = 0.0005). Weight loss was less in the early nutrition support group at time of discharge (mean: -0.4% ± 2.9% vs. -3.4% ± 2.6% in the usual care group, p = 0.001). This difference in weight was no longer observed at 6 months after discharge (mean: -1.0% ± 6.8% vs. 1.4% ± 6.1%, p = 0.29). In practice, an early start to nutrition support proved difficult because of patient resistance and physician preference, with 8 patients (33%) in the control group and 4 (15%) in the intervention group not commencing nutrition support when stipulated by the study protocol. No significant differences between the groups were found for other outcomes. In well-nourished patients receiving asct, early nutrition support maintained weight during admission, but did not affect other outcomes. Interpretation of results should take into consideration the difficulties encountered with intervention implementation.
Keywords: Nutrition; autologous stem-cell transplantation; enteral nutrition; malnutrition; parenteral nutrition.
Figures
References
-
- Sonis ST, Oster G, Fuchs H, et al. Oral mucositis and the clinical and economic outcomes of haematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–5. - PubMed
-
- Hung YC, Bauer J, Horsley P, Waterhouse M, Bashford J, Isenring E. Changes in nutritional status, body composition, quality of life, and physical activity levels of cancer patients undergoing autologous peripheral blood stem cell transplantation. Support Care Cancer. 2013;21:1579–86. doi: 10.1007/s00520-012-1698-y. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

