Poly[tetrakis(μ-1,1,1,3,3,3-hexafluoropropan-2-olato)iron(II)dipotassium]
- PMID: 24764807
- PMCID: PMC3998246
- DOI: 10.1107/S1600536813034946
Poly[tetrakis(μ-1,1,1,3,3,3-hexafluoropropan-2-olato)iron(II)dipotassium]
Abstract
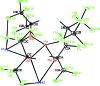
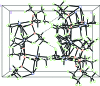
The title compound, [K2Fe{OCH(CF3)2}4] n , was formed from the reaction of potassium hexa-fluoro-isopropoxide with iron(II) chloride in toluene. The Fe(II) atom has a highly distorted tetra-hedral coordination environment. All four of the non-equivalent hexa-fluoro-isoprop-oxy O atoms link the Fe(II) atoms to one of the K(+) atoms in an alternating chain of Fe-O-K-O fused four-membered rings, with K-Fe distances of 3.715 (2) and 3.717 (2) Å. This K(+) atom is also bridged to eight of the F atoms. The other K(+) atom is bonded to only two of the O atoms, but has seven short K⋯F contacts, one of which links the chains into a three-dimensional arrangement. Weak hydrogen bonding between the lone H atoms on the hexa-fluoro-isoprop-oxy groups and F atoms is also present. The crystal studied was refined as an inversion twin.
Figures
References
-
- Agilent (2012). CrysAlis PRO Agilent Technologies, Yarnton, UK.
-
- Bernhardt, E., Brauer, D. J., Köckerling, M. & Pawelke, G. (2007). Z. Anorg. Allg. Chem. 633, 947–954.
-
- Cantalupo, S. A., Fiedler, S. R., Shores, M. P., Rheingold, A. L. & Doerrer, L. H. (2012). Angew. Chem. Int. Ed. 51, 1000–1005. - PubMed
-
- Cantalupo, S. A., Lum, J. S., Buzzeo, M. C., Moore, C., DiPasquale, A. G., Rheingold, A. L. & Doerrer, L. H. (2010). Dalton Trans. 39, 374–383. - PubMed
-
- Konefal, E., Loeb, S. J., Willis, C. J. & Stephan, D. W. (1986). Inorg. Chim. Acta, 115, 147–151.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials