1-Piperonylpiperazinium 4-nitro-benzoate monohydrate
- PMID: 24764985
- PMCID: PMC3998489
- DOI: 10.1107/S160053681400261X
1-Piperonylpiperazinium 4-nitro-benzoate monohydrate
Abstract
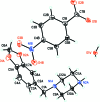
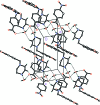
IN THE TITLE HYDRATED SALT [SYSTEMATIC NAME: 1-(1,3-benzodioxol-5-ylmeth-yl)piperazin-1-ium 4-nitro-benzoate monohydrate], C12H17N2O2 (+)·C7H4NO4 (-)·H2O, the piperazinium ring of the cation adopts a slightly distorted chair conformation. The piperonyl and piperazine rings are rotated with respect to each other with an N-C-C-C torsion angle of 45.6 (2)°. In the anion, the nitro group is almost coplanar with the adjacent benzene ring, forming a dihedral angle of only 3.9 (4)°. In the crystal, the cations, anions and water mol-ecules are linked through N-H⋯O and O-H⋯O hydrogen bonds into chains along the a axis. In addition, weaker inter-molecular C-H⋯O inter-actions are also observed within the chains. The anions form centrosymmetric couples through π-stacking inter-actions, with an inter-centroid distance of 3.681 (4) Å between the benzene rings.
Figures
References
-
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Brockunier, L. L., He, J., Colwell, L. F. Jr, Habulihaz, B., He, H., Leiting, B., Lyons, K. A., Marsilio, F., Patel, R. A., Teffera, Y., Wu, J. K., Thornberry, N. A., Weber, A. E. & Parmee, E. R. (2004). Bioorg. Med. Chem. Lett. 14, 4763–4766. - PubMed
-
- Capuano, B., Crosby, I. T., Gable, R. W. & Lloyd, E. J. (2000). Acta Cryst. C56, 339–340. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
