2-(4,5-Dimeth-oxy-2-nitro-phen-yl)-4-meth-oxy-3-methyl-9-phenyl-sulfonyl-9H-carbazole
- PMID: 24765031
- PMCID: PMC3998409
- DOI: 10.1107/S1600536814003535
2-(4,5-Dimeth-oxy-2-nitro-phen-yl)-4-meth-oxy-3-methyl-9-phenyl-sulfonyl-9H-carbazole
Abstract
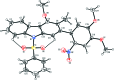
In the title compound, C28H24N2O7S, the carbazole system is essentially planar, with a maximum deviation of 0.0644 (19) Å for the C atom connected to the 4,5-dimeth-oxy-2-nitro-phenyl group. The dihedral angle between the carbazole moiety and the dimethoxy-substituted nitrophenyl ring is 58.55 (7)°. The sulfonyl group forms two intra-molecular C-H⋯O bonds with the adjacent carbazole system, forming two cyclic S(6) motifs. In the crystal, mol-ecules are linked along the a axis in bands consisting of cyclic R 3 (3)(15) motifs through two further C-H⋯O hydrogen bonds.
Figures

References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Bassindale, A. (1984). The Third Dimension in Organic Chemistry, ch. 1, p. 11. New York: John Wiley and Sons.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Other Literature Sources
