Social learning in humans and other animals
- PMID: 24765063
- PMCID: PMC3982061
- DOI: 10.3389/fnins.2014.00058
Social learning in humans and other animals
Abstract
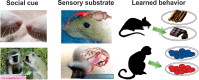
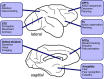
Decisions made by individuals can be influenced by what others think and do. Social learning includes a wide array of behaviors such as imitation, observational learning of novel foraging techniques, peer or parental influences on individual preferences, as well as outright teaching. These processes are believed to underlie an important part of cultural variation among human populations and may also explain intraspecific variation in behavior between geographically distinct populations of animals. Recent neurobiological studies have begun to uncover the neural basis of social learning. Here we review experimental evidence from the past few decades showing that social learning is a widespread set of skills present in multiple animal species. In mammals, the temporoparietal junction, the dorsomedial, and dorsolateral prefrontal cortex, as well as the anterior cingulate gyrus, appear to play critical roles in social learning. Birds, fish, and insects also learn from others, but the underlying neural mechanisms remain poorly understood. We discuss the evolutionary implications of these findings and highlight the importance of emerging animal models that permit precise modification of neural circuit function for elucidating the neural basis of social learning.
Keywords: DLPFC; anterior cingulate cortex; anterior cingulate gyrus; dorsolateral prefrontal cortex; learning; social; superior temporal sulcus; temporoparietal junction.
Figures

References
-
- Avery M. L. (1994). Finding good food and avoiding bad food – does it help to associated with experienced flockmates? Anim. Behav. 48, 1371–1378 10.1006/anbe.1994.1373 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

