A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging
- PMID: 24765076
- PMCID: PMC3980114
- DOI: 10.3389/fphar.2014.00056
A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging
Erratum in
-
Corrigendum: A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging.Front Pharmacol. 2016 Feb 25;7:46. doi: 10.3389/fphar.2016.00046. eCollection 2016. Front Pharmacol. 2016. PMID: 26941646 Free PMC article.
Abstract
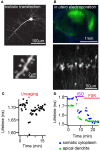
Neuromodulators have profound effects on behavior, but the dynamics of their intracellular effectors has remained unclear. Most neuromodulators exert their function via G-protein-coupled receptors (GPCRs). One major challenge for understanding neuromodulator action is the lack of dynamic readouts of the biochemical signals produced by GPCR activation. The adenylate cyclase/cyclic AMP/protein kinase A (PKA) module is a central component of such biochemical signaling. This module is regulated by several behaviorally important neuromodulator receptors. Furthermore, PKA activity is necessary for the induction of many forms of synaptic plasticity as well as for the formation of long-term memory. In order to monitor PKA activity in brain tissue, we have developed a 2-photon fluorescence lifetime imaging microscopy (2pFLIM) compatible PKA sensor termed FLIM-AKAR, which is based on the ratiometric FRET sensor AKAR3. FLIM-AKAR shows a large dynamic range and little pH sensitivity. In addition, it is a rapidly diffusible cytoplasmic protein that specifically reports net PKA activity in situ. FLIM-AKAR expresses robustly in various brain regions with multiple transfection methods, can be targeted to genetically identified cell types, and responds to activation of both endogenous GPCRs and spatial-temporally specific delivery of glutamate. Initial experiments reveal differential regulation of PKA activity across subcellular compartments in response to neuromodulator inputs. Therefore, the reporter FLIM-AKAR, coupled with 2pFLIM, enables the study of PKA activity in response to neuromodulator inputs in genetically identified neurons in the brain, and sheds light on the intracellular dynamics of endogenous GPCR activation.
Keywords: FLIM; FLIM-AKAR; GPCR; PKA; cAMP; dendritic spine; glutamate; neuromodulation.
Figures






References
-
- Ashby C. D., Walsh D. A. (1972). Characterization of the interaction of a protein inhibitor with adenosine 3′,5′-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J. Biol. Chem. 247, 6637–6642 - PubMed
-
- Ashby C. D., Walsh D. A. (1973). Characterization of the interaction of a protein inhibitor with adenosine 3′,5′-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J. Biol. Chem. 248, 1255–1261 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

