Circulating tumor cells as a prognostic factor in patients with small cell lung cancer
- PMID: 24765158
- PMCID: PMC3997694
- DOI: 10.3892/ol.2014.1940
Circulating tumor cells as a prognostic factor in patients with small cell lung cancer
Abstract
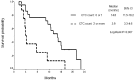
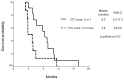
The detection of circulating tumor cells (CTCs) in peripheral blood is currently an important field of study. Detection of CTCs by the OBP-401 assay (TelomeScan®) has previously been reported to be useful in the diagnosis, prognosis and evaluation of therapeutic efficacy in breast and gastric cancer. The aim of the present study was to evaluate the OBP-401 assay as a novel method of detecting CTCs of small cell lung cancer (SCLC) patients and to evaluate whether CTC count is associated with prognosis. Prospectively, 30 consecutively diagnosed SCLC patients who had commenced chemotherapy or chemoradiotherapy were enrolled as subjects of the current study. Peripheral blood specimens were collected from the SCLC patients prior to and following the initiation of treatment and the viable CTCs were detected in the specimens following incubation with a telomerase-specific, replication-selective, oncolytic adenoviral agent, which was carrying the green fluorescent protein gene. CTCs were detected in 29 patients (96%). The group of 21 patients with a CTC count of <2 cells/7.5 ml prior to treatment (baseline) had a significantly longer median survival time than the group of eight patients with a CTC count of ≥2 cells/7.5 ml prior to treatment (14.8 and 3.9 months, respectively; P=0.007). The results of a multivariate analysis showed that the baseline CTC count was an independent prognostic factor for survival time (hazard ratio, 3.91; P=0.026). Among the patients that achieved a partial response to treatment, patients who had a CTC count of <2 cells/7.5 ml following two cycles of chemotherapy tended to have a longer median progression-free survival compared with patients who had a CTC count of ≥2 cell/7.5 ml (8.3 and 3.8 months, respectively; P=0.07). Therefore, CTCs may be detected via OBP-401 assay in SCLC patients and the CTC count prior to treatment appears to be a strong prognostic factor.
Keywords: OBP-401 assay; circulating tumor cells; prognostic factor; small cell lung cancer.
Figures

Similar articles
-
Predictive and prognostic value of folate receptor-positive circulating tumor cells in small cell lung cancer patients treated with first-line chemotherapy.Oncotarget. 2017 Jul 25;8(30):49044-49052. doi: 10.18632/oncotarget.17039. Oncotarget. 2017. PMID: 28467771 Free PMC article.
-
Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients.Oncotarget. 2017 May 23;8(21):34884-34895. doi: 10.18632/oncotarget.16818. Oncotarget. 2017. PMID: 28432274 Free PMC article.
-
Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer.Lung Cancer. 2014 Aug;85(2):314-9. doi: 10.1016/j.lungcan.2014.05.002. Epub 2014 May 14. Lung Cancer. 2014. PMID: 24882383
-
Significance of circulating tumor cells in lung cancer: a narrative review.Transl Lung Cancer Res. 2023 Apr 28;12(4):877-894. doi: 10.21037/tlcr-22-712. Epub 2023 Mar 29. Transl Lung Cancer Res. 2023. PMID: 37197632 Free PMC article. Review.
-
Promising Role of Circulating Tumor Cells in the Management of SCLC.Cancers (Basel). 2021 Apr 22;13(9):2029. doi: 10.3390/cancers13092029. Cancers (Basel). 2021. PMID: 33922300 Free PMC article. Review.
Cited by
-
Circulating tumour cells in patients with lung cancer universally indicate poor prognosis.Eur Respir Rev. 2022 Dec 14;31(166):220151. doi: 10.1183/16000617.0151-2022. Print 2022 Dec 31. Eur Respir Rev. 2022. PMID: 36517047 Free PMC article. Review.
-
Liquid biopsy in pancreatic ductal adenocarcinoma: current status of circulating tumor cells and circulating tumor DNA.Mol Oncol. 2019 Aug;13(8):1623-1650. doi: 10.1002/1878-0261.12537. Epub 2019 Jul 30. Mol Oncol. 2019. PMID: 31243883 Free PMC article. Review.
-
Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study.Invest New Drugs. 2017 Jun;35(3):334-344. doi: 10.1007/s10637-017-0446-z. Epub 2017 Mar 15. Invest New Drugs. 2017. PMID: 28299514 Free PMC article. Clinical Trial.
-
Prospective Exploratory Study of the Clinical Significance of Circulating Tumor Cells in Patients With Small Cell Lung Cancer Exposed to Prophylactic Cranial Irradiation.Front Oncol. 2021 Feb 8;10:575394. doi: 10.3389/fonc.2020.575394. eCollection 2020. Front Oncol. 2021. PMID: 33628727 Free PMC article.
-
Detection and preliminary evaluation of circulating tumor cells in the peripheral blood of patients with eight types of cancer using a telomerase-specific adenovirus.Oncol Rep. 2014 Nov;32(5):1772-8. doi: 10.3892/or.2014.3436. Epub 2014 Aug 22. Oncol Rep. 2014. PMID: 25176113 Free PMC article.
References
-
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. - PubMed
-
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. - PubMed
-
- Turrisi AT, III, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. - PubMed
-
- Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. - PubMed
-
- Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013–5021. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
