Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor proliferation, migration and invasion of ovarian cancer cell lines
- PMID: 24765180
- PMCID: PMC3997722
- DOI: 10.3892/ol.2014.1926
Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor proliferation, migration and invasion of ovarian cancer cell lines
Abstract
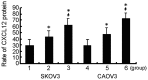
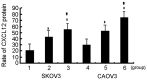
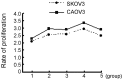
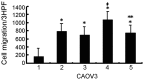
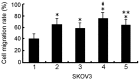
Ovarian cancer is the most fatal gynecological cancer, with a 5-year survival rate of only 30%. Lysophosphatidic acid (LPA), which possesses growth factor-like functions, is a major regulatory factor in the peritoneal metastasis of ovarian cancer. LPA stimulates the expression of numerous genes that are associated with angiogenesis and metastasis. Ovarian epithelial carcinoma specifically expresses chemotactic factor C-X-C motif chemokine ligand 12 (CXCL12) and its receptor, CXC receptor 4 (CXCR4). The CXCL12-CXCR4 axis directly contributes to ovarian cancer cell proliferation, migration and invasion. The present study investigated the regulation of LPA on the CXCL12-CXCR4 axis and the effect of the LPA-mediated CXCL12-CXCR4 axis on the tumor proliferation, migration and invasion of ovarian cancer cell lines. The CXCR4 proteins expressed in the cell membrane and the cytoplasm of ovarian cancer cells, CAOV3 and SKOV3, were detected by immunocytochemistry. The expression of CXCR4 and CXCL12 was increased in the ovarian cancer cells in a dose- and time-dependent manner when treated with LPA compared with the control groups (P<0.05), as determined by reverse transcription polymerase chain reaction and flow cytometry. LPA (20 μM) and CXCL12 (100 ng/ml) enhanced the proliferation, migration and invasion of the ovarian cancer cells, CAOV3 and SKOV3, as identified by MTT, Transwell and Matrigel assays following co-treatment for 24 h. LPA promoted invasiveness of ovarian cancer by upregulating CXCL12-CXCR4 axis expression.
Keywords: CXCL12-CXCR4 axis; lysophosphatidic acid; metastasis; ovarian neoplasm.
Figures



Similar articles
-
[Effect of chemokine CXCL12 and its receptor CXCR4 on proliferation, migration and invasion of epithelial ovarian cancer cells].Zhonghua Fu Chan Ke Za Zhi. 2007 Jun;42(6):403-7. Zhonghua Fu Chan Ke Za Zhi. 2007. PMID: 17697603 Chinese.
-
CXCL12-CXCR4 Axis Promotes Proliferation, Migration, Invasion, and Metastasis of Ovarian Cancer.Oncol Res. 2014;22(5-6):247-58. doi: 10.3727/096504015X14343704124430. Oncol Res. 2014. PMID: 26629936 Free PMC article.
-
Role of CXCL12 in metastasis of human ovarian cancer.Chin Med J (Engl). 2007 Jul 20;120(14):1251-5. Chin Med J (Engl). 2007. PMID: 17697577
-
The chemokine receptors CXCR4/CXCR7 and their primary heterodimeric ligands CXCL12 and CXCL12/high mobility group box 1 in pancreatic cancer growth and development: finding flow.Pancreas. 2015 May;44(4):528-34. doi: 10.1097/MPA.0000000000000298. Pancreas. 2015. PMID: 25872129 Review.
-
C-X-C motif chemokine ligand 12-C-X-C chemokine receptor type 4 signaling axis in cancer and the development of chemotherapeutic molecules.Tzu Chi Med J. 2024 May 27;36(3):231-239. doi: 10.4103/tcmj.tcmj_52_24. eCollection 2024 Jul-Sep. Tzu Chi Med J. 2024. PMID: 38993827 Free PMC article. Review.
Cited by
-
PLA2G16 promotes osteosarcoma metastasis and drug resistance via the MAPK pathway.Oncotarget. 2016 Apr 5;7(14):18021-35. doi: 10.18632/oncotarget.7694. Oncotarget. 2016. PMID: 26933804 Free PMC article.
-
Pharmacological activation of lysophosphatidic acid receptors regulates erythropoiesis.Sci Rep. 2016 May 31;6:27050. doi: 10.1038/srep27050. Sci Rep. 2016. PMID: 27244685 Free PMC article.
-
Extracellular HSP70/HSP70-PCs regulate hepatocarcinoma cell migration and invasion via RhoA.Oncol Lett. 2017 Mar;13(3):1095-1100. doi: 10.3892/ol.2016.5551. Epub 2016 Dec 30. Oncol Lett. 2017. PMID: 28454219 Free PMC article.
-
Impact of tumor necrosis factor-alpha and lysophosphatidic acid on the behavior of ovarian cancer cells in a three-dimensional collagen hydrogel.J Obstet Gynaecol Res. 2025 Aug;51(8):e70026. doi: 10.1111/jog.70026. J Obstet Gynaecol Res. 2025. PMID: 40754666 Free PMC article.
-
CXCL12 chemokine expression suppresses human breast cancer growth and metastasis in vitro and in vivo.Int J Clin Exp Pathol. 2014 Sep 15;7(10):6671-8. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400746 Free PMC article.
References
-
- Xu Y, Gaudette DC, Boynton JD, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. - PubMed
-
- Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. - PubMed
-
- Hu YL, Albanese C, Pestell RG, Jaffe RB. Dual mechanisms for lysophosphatidic acid stimulation of human ovarian carcinoma cells. J Natl Cancer Inst. 2003;95:733–740. - PubMed
-
- Frankel A, Mills GB. Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clin Cancer Res. 1996;2:1307–1313. - PubMed
-
- Pustilnik TB, Estrella V, Wiener JR, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
