Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: focus on future developments
- PMID: 24765618
- PMCID: PMC3991004
- DOI: 10.1007/s40336-014-0054-2
Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: focus on future developments
Abstract
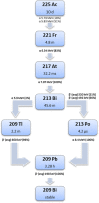
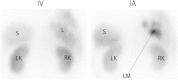
Peptide receptor radionuclide therapy (PRRT) has been shown to be an effective treatment for neuroendocrine tumors (NETs) if curative surgery is not an option. A majority of NETs abundantly express somatostatin receptors. Consequently, following administration of somatostatin (SST) analogs labeled with γ-emitting radionuclides, these tumors can be imaged for diagnosis, staging or follow-up purposes. Furthermore, when β-emitting radionuclides are used, radiolabeled peptides (radiopeptides) can also be used for the treatment for NET patients. Even though excellent results have been achieved with PRRT, complete responses are still rare, which means that there is room for improvement. In this review, we highlight some of the directions currently under investigation in pilot clinical studies or in preclinical development to achieve this goal. Although randomized clinical trials are still lacking, early studies have shown that tumor response might be improved by application of other radionuclides, such as α-emitters or radionuclide combinations, or by adjustment of radiopeptide administration routes. Individualized dosimetry and better insight into tumor and normal organ radiation doses may allow adjustment of the amount of administered activity per cycle or the number of treatment cycles, resulting in more personalized treatment schedules. Other options include the application of novel (radiolabeled) SST analogs with improved tumor uptake and radionuclide retention time, or a combination of PRRT with other systemic therapies, such as chemotherapy or treatment with radio sensitizers. Though promising directions appear to bring improvements of PRRT within reach, additional research (including randomized clinical trials) is needed to achieve such improvements.
Keywords: Dosimetry; Neuroendocrine tumor; Peptide receptor radionuclide therapy; Somatostatin analogs.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
