Multiple redundant medulla projection neurons mediate color vision in Drosophila
- PMID: 24766346
- PMCID: PMC4245076
- DOI: 10.3109/01677063.2014.891590
Multiple redundant medulla projection neurons mediate color vision in Drosophila
Abstract
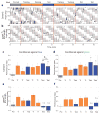
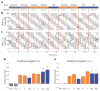
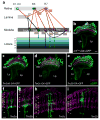
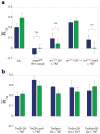
The receptor mechanism for color vision has been extensively studied. In contrast, the circuit(s) that transform(s) photoreceptor signals into color percepts to guide behavior remain(s) poorly characterized. Using intersectional genetics to inactivate identified subsets of neurons, we have uncovered the first-order interneurons that are functionally required for hue discrimination in Drosophila. We developed a novel aversive operant conditioning assay for intensity-independent color discrimination (true color vision) in Drosophila. Single flying flies are magnetically tethered in an arena surrounded by blue and green LEDs (light-emitting diodes). The flies' optomotor response is used to determine the blue-green isoluminant intensity. Flies are then conditioned to discriminate between equiluminant blue or green stimuli. Wild-type flies are successfully trained in this paradigm when conditioned to avoid either blue or green. Functional color entrainment requires the function of the narrow-spectrum photoreceptors R8 and/or R7, and is within a limited range, intensity independent, suggesting that it is mediated by a color vision system. The medulla projection neurons, Tm5a/b/c and Tm20, receive direct inputs from R7 or R8 photoreceptors and indirect input from the broad-spectrum photoreceptors R1-R6 via the lamina neuron L3. Genetically inactivating these four classes of medulla projection neurons abolished color learning. However, inactivation of subsets of these neurons is insufficient to block color learning, suggesting that true color vision is mediated by multiple redundant pathways. We hypothesize that flies represent color along multiple axes at the first synapse in the fly visual system. The apparent redundancy in learned color discrimination sharply contrasts with innate ultraviolet (UV) spectral preference, which is dominated by a single pathway from the amacrine neuron Dm8 to the Tm5c projection neurons.
Keywords: color discrimination; medulla projection neurons; neural substrate; visual behavior.
Figures

References
-
- Borst A. Drosophila’s view on insect vision. Curr Biol. 2009;19:R36–47. - PubMed
-
- Bender JA, Dickinson MH. Visual stimulation of saccades in magnetically tethered Drosophila. J Exp Biol. 2006;209(Pt 16):3170–3182. - PubMed
-
- Cajal SR, Sanchez D. Contribucion al conocimiento de los centros nerviosos del los insectos. Trab Lab Invest Biol. 1915;13:1–167.
-
- Calkins DJ, Sterling P. Evidence that circuits for spatial and color vision segregate at the first retinal synapse. Neuron. 1999;24:313–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
