WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells
- PMID: 24766647
- PMCID: PMC4022450
- DOI: 10.1186/1476-4598-13-88
WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells
Abstract
Background: Wnt proteins are important for developmental processes and certain diseases. WNT5A is a non-canonical Wnt protein that previously has been shown to play a role in the progression of malignant melanoma. High expression of WNT5A in melanoma tumors correlates to formation of distant metastasis and poor prognosis. This has partly been described by the findings that WNT5A expression in melanoma cell lines increases migration and invasion.
Methods: Malignant melanoma cell lines were treated with rWNT5A or WNT5A siRNA, and mRNA versus protein levels of soluble mediators were measured using RT-PCR, cytokine bead array and ELISA. The induced signaling pathways were analyzed using inhibitors, Rho-GTPase pull down assays and western blot. Ultracentrifugation and electron microscopy was used to analyze microvesicles. Gene expression microarray data obtained from primary malignant melanomas was used to verify our data.
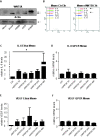
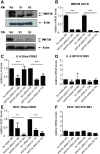
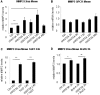
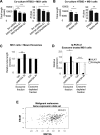
Results: We show that WNT5A signaling induces a Ca2+-dependent release of exosomes containing the immunomodulatory and pro-angiogenic proteins IL-6, VEGF and MMP2 in melanoma cells. The process was independent of the transcriptional machinery and depletion of WNT5A reduced the levels of the exosome-derived proteins. The WNT5A induced exosomal secretion was neither affected by Tetanus toxin nor Brefeldin A, but was blocked by the calcium chelator Bapta, inhibited by a dominant negative version of the small Rho-GTPase Cdc42 and was accompanied by cytoskeletal reorganization. Co-cultures of melanoma/endothelial cells showed that depletion of WNT5A in melanoma cells decreased endothelial cell branching, while stimulation of endothelial cells with isolated rWNT5A-induced melanoma exosomes increased endothelial cell branching in vitro. Finally, gene expression data analysis of primary malignant melanomas revealed a correlation between WNT5A expression and the angiogenesis marker ESAM.
Conclusions: These data indicate that WNT5A has a broader function on tumor progression and metastatic spread than previously known; by inducing exosome-release of immunomodulatory and pro-angiogenic factors that enhance the immunosuppressive and angiogenic capacity of the tumors thus rendering them more aggressive and more prone to metastasize.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

