Mosquitoes rely on their gut microbiota for development
- PMID: 24766707
- PMCID: PMC4083365
- DOI: 10.1111/mec.12771
Mosquitoes rely on their gut microbiota for development
Abstract
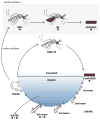
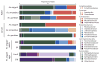
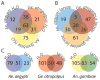
Field studies indicate adult mosquitoes (Culicidae) host low diversity communities of bacteria that vary greatly among individuals and species. In contrast, it remains unclear how adult mosquitoes acquire their microbiome, what influences community structure, and whether the microbiome is important for survival. Here, we used pyrosequencing of 16S rRNA to characterize the bacterial communities of three mosquito species reared under identical conditions. Two of these species, Aedes aegypti and Anopheles gambiae, are anautogenous and must blood-feed to produce eggs, while one, Georgecraigius atropalpus, is autogenous and produces eggs without blood feeding. Each mosquito species contained a low diversity community comprised primarily of aerobic bacteria acquired from the aquatic habitat in which larvae developed. Our results suggested that the communities in Ae. aegypti and An. gambiae larvae share more similarities with one another than with G. atropalpus. Studies with Ae. aegypti also strongly suggested that adults transstadially acquired several members of the larval bacterial community, but only four genera of bacteria present in blood fed females were detected on eggs. Functional assays showed that axenic larvae of each species failed to develop beyond the first instar. Experiments with Ae. aegypti indicated several members of the microbial community and Escherichia coli successfully colonized axenic larvae and rescued development. Overall, our results provide new insights about the acquisition and structure of bacterial communities in mosquitoes. They also indicate that three mosquito species spanning the breadth of the Culicidae depend on their gut microbiome for development.
Keywords: bacteria; development; evolution; insects; microbial biology.
© 2014 John Wiley & Sons Ltd.
Figures



References
-
- Beck M, Strand MR. RNA interference silences Microplitis demolitor bracovirus genes and implicates glc1.8 in disruption of adhesion in infected host cells. Virology. 2003;314:521–535. - PubMed
-
- Briegel H. Physiological bases of mosquito ecology. Journal of Vector Ecology. 2003;28:1–11. - PubMed
-
- Briones AM, Shililu J, Githure J, Novak R, Raskin L. Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. ISME Journal. 2008;2:74–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

