Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region
- PMID: 24766735
- PMCID: PMC4022269
- DOI: 10.1186/1756-3305-7-199
Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region
Abstract
Background: Disease risk maps are important tools that help ascertain the likelihood of exposure to specific infectious agents. Understanding how climate change may affect the suitability of habitats for ticks will improve the accuracy of risk maps of tick-borne pathogen transmission in humans and domestic animal populations. Lyme disease (LD) is the most prevalent arthropod borne disease in the US and Europe. The bacterium Borrelia burgdorferi causes LD and it is transmitted to humans and other mammalian hosts through the bite of infected Ixodes ticks. LD risk maps in the transboundary region between the U.S. and Mexico are lacking. Moreover, none of the published studies that evaluated the effect of climate change in the spatial and temporal distribution of I. scapularis have focused on this region.
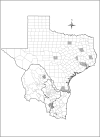
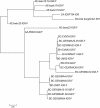
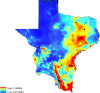
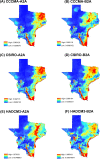
Methods: The area of study included Texas and a portion of northeast Mexico. This area is referred herein as the Texas-Mexico transboundary region. Tick samples were obtained from various vertebrate hosts in the region under study. Ticks identified as I. scapularis were processed to obtain DNA and to determine if they were infected with B. burgdorferi using PCR. A maximum entropy approach (MAXENT) was used to forecast the present and future (2050) distribution of B. burgdorferi-infected I. scapularis in the Texas-Mexico transboundary region by correlating geographic data with climatic variables.
Results: Of the 1235 tick samples collected, 109 were identified as I. scapularis. Infection with B. burgdorferi was detected in 45% of the I. scapularis ticks collected. The model presented here indicates a wide distribution for I. scapularis, with higher probability of occurrence along the Gulf of Mexico coast. Results of the modeling approach applied predict that habitat suitable for the distribution of I. scapularis in the Texas-Mexico transboundary region will remain relatively stable until 2050.
Conclusions: The Texas-Mexico transboundary region appears to be part of a continuum in the pathogenic landscape of LD. Forecasting based on climate trends provides a tool to adapt strategies in the near future to mitigate the impact of LD related to its distribution and risk for transmission to human populations in the Mexico-US transboundary region.
Figures

Comment in
-
Analysis of the intergenic sequences provided by Feria-Arroyo et al. does not support the claim of high Borrelia burgdorferi tick infection rates in Texas and northeastern Mexico.Parasit Vectors. 2014 Oct 21;7(1):467. doi: 10.1186/s13071-014-0467-9. Parasit Vectors. 2014. PMID: 25428816 Free PMC article. No abstract available.
-
Response to Esteve-Gassent et al.: flaB sequences obtained from Texas PCR products are identical to the positive control strain Borrelia burgdorferi B31.Parasit Vectors. 2015 Jun 9;8:310. doi: 10.1186/s13071-015-0899-x. Parasit Vectors. 2015. PMID: 26050617 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

