Bacteria fighting back: how pathogens target and subvert the host innate immune system
- PMID: 24766896
- PMCID: PMC4023866
- DOI: 10.1016/j.molcel.2014.03.010
Bacteria fighting back: how pathogens target and subvert the host innate immune system
Abstract
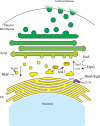
The innate immune system has evolved under selective pressure since the radiation of multicellular life approximately 600 million years ago. Because of this long history, innate immune mechanisms found in modern eukaryotic organisms today are highly complex but yet built from common molecular strategies. It is now clear that evolution has selected a conserved set of antimicrobial peptides as well as pattern-recognition receptors (PRRs) that initiate cellular-based signals as a first line of defense against invading pathogens. Conversely, microbial pathogens employ their own strategies in order to evade, inhibit, or otherwise manipulate the innate immune response. Here, we discuss recent discoveries that have changed our view of immune modulatory mechanisms employed by bacterial pathogens, focusing specifically on the initial sites of microbial recognition and extending to host cellular signal transduction, proinflammatory cytokine production, and alteration of protein trafficking and secretion.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

