Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia
- PMID: 24767094
- PMCID: PMC4101869
- DOI: 10.1186/1741-7015-12-67
Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia
Abstract
Background: Severe anemia (SA, hemoglobin <6 g/dl) is a leading cause of pediatric hospital admission in Africa, with significant in-hospital mortality. The underlying etiology is often infectious, but specific pathogens are rarely identified. Guidelines developed to encourage rational blood use recommend a standard volume of whole blood (20 ml/kg) for transfusion, but this is commonly associated with a frequent need for repeat transfusion and poor outcome. Evidence is lacking on what hemoglobin threshold criteria for intervention and volume are associated with the optimal survival outcomes.
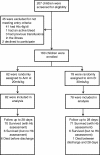
Methods: We evaluated the safety and efficacy of a higher volume of whole blood (30 ml/kg; Tx30: n = 78) against the standard volume (20 ml/kg; Tx20: n = 82) in Ugandan children (median age 36 months (interquartile range (IQR) 13 to 53)) for 24-hour anemia correction (hemoglobin >6 g/dl: primary outcome) and 28-day survival.
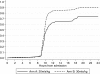
Results: Median admission hemoglobin was 4.2 g/dl (IQR 3.1 to 4.9). Initial volume received followed the randomization strategy in 155 (97%) patients. By 24-hours, 70 (90%) children in the Tx30 arm had corrected SA compared to 61 (74%) in the Tx20 arm; cause-specific hazard ratio = 1.54 (95% confidence interval 1.09 to 2.18, P = 0.01). From admission to day 28 there was a greater hemoglobin increase from enrollment in Tx30 (global P <0.0001). Serious adverse events included one non-fatal allergic reaction and one death in the Tx30 arm. There were six deaths in the Tx20 arm (P = 0.12); three deaths were adjudicated as possibly related to transfusion, but none secondary to volume overload.
Conclusion: A higher initial transfusion volume prescribed at hospital admission was safe and resulted in an accelerated hematological recovery in Ugandan children with SA. Future testing in a large, pragmatic clinical trial to establish the effect on short and longer-term survival is warranted.
Trial registration: ClinicalTrials.Gov identifier: NCT01461590 registered 26 October 2011.
Figures

Comment in
-
Risks and benefits of transfusion for children with severe anemia in Africa.BMC Med. 2014 Apr 25;12:68. doi: 10.1186/1741-7015-12-68. BMC Med. 2014. PMID: 24767140 Free PMC article.
References
-
- de Benoist B, McLean E, Egli I, Cogswell M, Worldwide prevalence of anaemia 1993–2005. WHO global database on anaemia. In WHO report. 2008. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf [accessed 31 March 2014] (Geneva)
-
- Ala F, Allain JP, Bates I, Boukef K, Boulton F, Brandful J, Dax EM, El Ekiaby M, Farrugia A, Gorlin J, Hassall O, Lee H, Loua A, Maitland K, Mbanya D, Mukhtar Z, Murphy W, Opare-Sem O, Owusu-Ofori S, Reesink H, Roberts D, Torres O, Totoe G, Ullum H, Wendel S. External financial aid to blood transfusion services in sub-Saharan Africa: a need for reflection. PLoS Med. 2012;9:e1001309. doi: 10.1371/journal.pmed.1001309. - DOI - PMC - PubMed
-
- WHO Department of Blood Safety. Aide Memoire. In: Technology DoBSaC, editor. WHO/BCT/02.03. 1211 Geneva 27. Geneva: World Health Organization; 2002. pp. 1–2.
-
- WHO/FCH/CAH/00.01. Geneva: WHO: World Health Organization; 2000. Management of the child with a serious infection or severe malnutrition: guidelines for care at the first-referral level in developing countries.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

