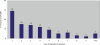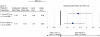Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis
- PMID: 24767095
- PMCID: PMC4018988
- DOI: 10.1186/1472-6963-14-189
Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis
Abstract
Background: To examine duration of daily filgrastim prophylaxis, and risk and consequences of chemotherapy-induced neutropenic complications (CINC) requiring inpatient care.
Methods: Using a retrospective cohort design and US healthcare claims data (2001-2010), we identified all cancer patients who initiated ≥1 course of myelosuppressive chemotherapy and received daily filgrastim prophylactically in ≥1 cycle. Cycles with daily filgrastim prophylaxis were pooled for analyses. CINC was identified based on hospital admissions with a diagnosis of neutropenia, fever, or infection; consequences were characterized in terms of hospital mortality, hospital length of stay (LOS), and CINC-related healthcare expenditures.
Results: Risk of CINC requiring inpatient care-adjusted for patient characteristics-was 2.4 (95% CI: 1.6-3.4) and 1.9 (1.3-2.8) times higher with 1-3 (N = 8371) and 4-6 (N = 3691) days of filgrastim prophylaxis, respectively, versus ≥7 days (N = 2226). Among subjects who developed CINC, consequences with 1-3 and 4-6 (vs. ≥7) days of filgrastim prophylaxis were: mortality (8.4% [n/N = 10/119] and 4.0% [3/75] vs. 0% [0/34]); LOS (means: 7.4 [N = 243] and 7.1 [N = 99] vs. 6.5 [N = 40]); and expenditures (means: $18,912 [N = 225] and $14,907 [N = 94] vs. $13,165 [N = 39]).
Conclusions: In this retrospective evaluation, shorter courses of daily filgrastim prophylaxis were found to be associated with an increased risk of CINC as well as poorer outcomes among those developing this condition. Because of the limitations inherent in healthcare claims databases specifically and retrospective evaluations generally, additional research addressing these limitations is needed to confirm the findings of this study.
Figures
Similar articles
-
Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF).BMC Cancer. 2013 Jan 8;13:11. doi: 10.1186/1471-2407-13-11. BMC Cancer. 2013. PMID: 23298389 Free PMC article.
-
Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy.Am J Clin Oncol. 2012 Jun;35(3):267-74. doi: 10.1097/COC.0b013e31820dc075. Am J Clin Oncol. 2012. PMID: 21378538
-
Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study.Curr Med Res Opin. 2011 Jan;27(1):79-86. doi: 10.1185/03007995.2010.536527. Epub 2010 Nov 22. Curr Med Res Opin. 2011. PMID: 21091127
-
Once-per-cycle pegfilgrastim (Neulasta) for the management of chemotherapy-induced neutropenia.Semin Oncol. 2003 Aug;30(4 Suppl 13):24-30. doi: 10.1016/s0093-7754(03)00314-2. Semin Oncol. 2003. PMID: 14508717 Review.
-
2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours.Eur J Cancer. 2011 Jan;47(1):8-32. doi: 10.1016/j.ejca.2010.10.013. Epub 2010 Nov 20. Eur J Cancer. 2011. PMID: 21095116
Cited by
-
Incidence of febrile neutropenia during chemotherapy among patients with nonmyeloid cancer receiving filgrastim vs a filgrastim biosimilar.Clinicoecon Outcomes Res. 2018 Sep 3;10:493-500. doi: 10.2147/CEOR.S168298. eCollection 2018. Clinicoecon Outcomes Res. 2018. PMID: 30214262 Free PMC article.
-
Clinical confirmation to demonstrate similarity for a biosimilar pegfilgrastim: a 3-way randomized equivalence study for a proposed biosimilar pegfilgrastim versus US-licensed and EU-approved reference products in breast cancer patients receiving myelosuppressive chemotherapy.Exp Hematol Oncol. 2018 Sep 6;7:22. doi: 10.1186/s40164-018-0114-9. eCollection 2018. Exp Hematol Oncol. 2018. PMID: 30202638 Free PMC article.
-
The Optimal Timing and Duration of Daily G-CSF for the Primary Prevention of Febrile Neutropenia in Breast Cancer Patients Undergoing Adjuvant TAC Chemotherapy.Asia Pac J Clin Oncol. 2025 Aug;21(4):383-391. doi: 10.1111/ajco.14165. Epub 2025 Mar 25. Asia Pac J Clin Oncol. 2025. PMID: 40130679 Free PMC article. Clinical Trial.
-
Risk of chemotherapy-induced febrile neutropenia in intermediate-risk regimens: Clinical and economic outcomes of granulocyte colony-stimulating factor prophylaxis.J Manag Care Spec Pharm. 2023 Feb;29(2):128-138. doi: 10.18553/jmcp.2023.29.2.128. J Manag Care Spec Pharm. 2023. PMID: 36705281 Free PMC article.
-
Cost-effectiveness of granulocyte colony-stimulating factors (G-CSFs) for the prevention of febrile neutropenia (FN) in patients with cancer.Support Care Cancer. 2023 Sep 20;31(10):581. doi: 10.1007/s00520-023-08043-4. Support Care Cancer. 2023. PMID: 37728795 Free PMC article.
References
-
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;14(19):3187–3205. doi: 10.1200/JCO.2006.06.4451. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources