Defining critical residues for substrate binding to 1-deoxy-D-xylulose 5-phosphate synthase--active site substitutions stabilize the predecarboxylation intermediate C2α-lactylthiamin diphosphate
- PMID: 24767541
- PMCID: PMC4065394
- DOI: 10.1111/febs.12823
Defining critical residues for substrate binding to 1-deoxy-D-xylulose 5-phosphate synthase--active site substitutions stabilize the predecarboxylation intermediate C2α-lactylthiamin diphosphate
Abstract
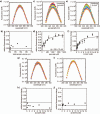
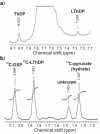
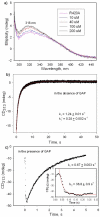
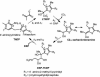
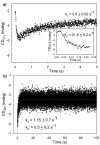
1-Deoxy-D-xylulose 5-phosphate (DXP) synthase catalyzes the formation of DXP from pyruvate and D-glyceraldehyde 3-phosphate (GraP) in a thiamin diphosphate-dependent manner, and is the first step in the essential pathway to isoprenoids in human pathogens. Understanding the mechanism of this unique enzyme is critical for developing new anti-infective agents that selectively target isoprenoid biosynthesis. The present study used mutagenesis and a combination of protein fluorescence, CD and kinetics experiments to investigate the roles of Arg420, Arg478 and Tyr392 in substrate binding and catalysis. The results support a random sequential, preferred order mechanism, and predict that Arg420 and Arg478 are involved in binding of the acceptor substrate, GraP. D-Glyceraldehyde, an alternative acceptor substrate lacking the phosphoryl group predicted to interact with Arg420 and Arg478, also accelerates decarboxylation of the predecarboxylation intermediate C2α-lactylthiamin diphosphate (LThDP) on DXP synthase, indicating that this binding interaction is not absolutely required, and that the hydroxyaldehyde sufficiently triggers decarboxylation. Unexpectedly, Tyr392 contributes to GraP affinity, and is not required for LThDP formation or its GraP-promoted decarboxylation. Time-resolved CD spectroscopy and NMR experiments indicate that LThDP is significantly stabilized on R420A and Y392F variants as compared with wild-type DXP synthase in the absence of acceptor substrate, but these substitutions do not appear to affect the rate of GraP-promoted LThDP decarboxylation in the presence of high levels of GraP, and LThDP formation remains the rate-limiting step. These results suggest a role of these residues in promoting GraP binding, which in turn facilitates decarboxylation, and also highlight interesting differences between DXP synthase and other thiamin diphosphate-dependent enzymes.
Keywords: CD; DXP synthase; enzymology; protein fluorescence; thiamin.
© 2014 FEBS.
Figures







References
-
- Sprenger GA, Schörken U, Wiegert T, Grolle S, de Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12857–12862. - PMC - PubMed
-
- Lois LM, Campos N, Putra SR, Danielsen K, Rohmer M, Boronat A. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2105–2110. - PMC - PubMed
-
- Himmeldirk K, Kennedy IA, Hill RE, Sayer BG, Spenser ID. Biosynthesis of vitamins B1 and B6 in Escherichia coli: Concurrent incorporation of 1-deoxy-d-xylulose into thiamin (B1) and pyridoxol (B6) Chem. Comm. 1996;271:1187–1188. - PubMed
-
- Hill RE, Himmeldirk K, Kennedy IA, Pauloski RM, Sayer BG, Wolf E, Spenser ID. The biogenetic anatomy of vitamin B6. A 13C NMR investigation of the biosynthesis of pyridoxol in Escherichia coli. J. Biol. Chem. 1996;271:30426–30435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

