Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia
- PMID: 24767576
- PMCID: PMC4013807
- DOI: 10.1186/1471-2377-14-91
Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia
Abstract
Background: Botulinum toxins are considered first-line therapy for treatment of cervical dystonia (CD) and must be injected on a repeat basis. Understanding the duration of clinical benefit of botulinum toxins and its impact on health care utilization are thus important in the contemporary environment. However, there is currently no overall consensus on the duration of effect of onabotulinumtoxinA in the treatment of CD. We performed a systematic review and meta-analysis to identify the duration of effect of onabotulinumtoxinA in CD and investigate factors that may influence it.
Methods: A systematic literature search identified prospective or retrospective studies reporting duration of effect of onabotulinumtoxinA for the treatment of CD. Inclusion criteria included peer-reviewed, non-review, English-language articles published between January 1980 and January 2013. A formal meta-analysis using Comprehensive Meta-Analysis Version 2 was conducted to identify the duration of effect of onabotulinumtoxinA in the treatment of CD; both fixed and random effects models were performed. Subgroup analyses were performed to identify factors that influenced the duration of effect of onabotulinumtoxinA.
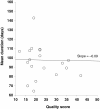
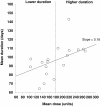
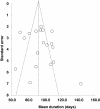
Results: A total of 18 studies (including >1,900 patients) met the inclusion criteria and were used for the meta-analysis. The mean duration of effect of onabotulinumtoxinA in CD was found to be 93.2 days (95% CI 91.8-94.6 days) for the fixed effects model and 95.2 days (95% CI 88.9-101.4 days) for the random effects model. A meta-regression found that dose of onabotulinumtoxinA and country of origin influenced the duration of effect of onabotulinumtoxinA, whereas quality score of the article and study type did not. In particular, doses ≥180 Units were associated with longer durations of effect than doses <180 Units (107-109 days vs. 86-88 days, respectively; p < 0.01). Limitations included pooling studies that used discrete definitions of duration and had different designs and study quality.
Conclusions: Based on the published literature, the mean duration of effect of onabotulinumtoxinA in CD was 93-95 days (13.2-13.5 weeks). This suggests that, in general, patients with CD treated with onabotulinumtoxinA should require ~4 treatments per year.
Figures
Similar articles
-
Botulinum toxin type A therapy for cervical dystonia.Cochrane Database Syst Rev. 2017 Dec 12;12(12):CD003633. doi: 10.1002/14651858.CD003633.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Nov 12;11:CD003633. doi: 10.1002/14651858.CD003633.pub4. PMID: 29230798 Free PMC article. Updated.
-
Botulinum toxin type A versus botulinum toxin type B for cervical dystonia.Cochrane Database Syst Rev. 2016 Oct 26;10(10):CD004314. doi: 10.1002/14651858.CD004314.pub3. Cochrane Database Syst Rev. 2016. PMID: 27782297 Free PMC article.
-
Botulinum toxin type B for cervical dystonia.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004315. doi: 10.1002/14651858.CD004315.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2016 May 13;(5):CD004315. doi: 10.1002/14651858.CD004315.pub3. PMID: 15674941 Updated.
-
Botulinum toxin type B for cervical dystonia.Cochrane Database Syst Rev. 2016 May 13;2016(5):CD004315. doi: 10.1002/14651858.CD004315.pub3. Cochrane Database Syst Rev. 2016. PMID: 27176573 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
Cited by
-
Striving for more good days: patient perspectives on botulinum toxin for the treatment of cervical dystonia.Patient Prefer Adherence. 2016 Aug 22;10:1601-8. doi: 10.2147/PPA.S106560. eCollection 2016. Patient Prefer Adherence. 2016. PMID: 27578965 Free PMC article.
-
Knowledge and practice of deep brain stimulation among pediatric neurology residents in Saudi Arabia.J Med Life. 2025 Feb;18(2):140-146. doi: 10.25122/jml-2025-0021. J Med Life. 2025. PMID: 40134440 Free PMC article.
-
Diagnosis and treatment of dystonia.Neurol Clin. 2015 Feb;33(1):77-100. doi: 10.1016/j.ncl.2014.09.002. Neurol Clin. 2015. PMID: 25432724 Free PMC article. Review.
-
Deep brain stimulation in pediatric dystonia: a systematic review.Neurosurg Rev. 2020 Jun;43(3):873-880. doi: 10.1007/s10143-018-1047-9. Epub 2018 Nov 5. Neurosurg Rev. 2020. PMID: 30397842 Free PMC article.
-
Patient considerations in the treatment of cervical dystonia: focus on botulinum toxin type A.Patient Prefer Adherence. 2015 Jun 2;9:725-31. doi: 10.2147/PPA.S75459. eCollection 2015. Patient Prefer Adherence. 2015. PMID: 26082621 Free PMC article. Review.
References
-
- BOTOX® [package insert] Irvine, CA: Allergan, Inc.; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous