Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike
- PMID: 24767986
- PMCID: PMC4109818
- DOI: 10.1016/j.celrep.2014.04.001
Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike
Abstract
Broadly neutralizing antibodies (bNAbs) to HIV-1 envelope glycoprotein (Env) can prevent infection in animal models. Characterized bNAb targets, although key to vaccine and therapeutic strategies, are currently limited. We defined a new site of vulnerability by solving structures of bNAb 8ANC195 complexed with monomeric gp120 by X-ray crystallography and trimeric Env by electron microscopy. The site includes portions of gp41 and N-linked glycans adjacent to the CD4-binding site on gp120, making 8ANC195 the first donor-derived anti-HIV-1 bNAb with an epitope spanning both Env subunits. Rather than penetrating the glycan shield by using a single variable-region CDR loop, 8ANC195 inserted its entire heavy-chain variable domain into a gap to form a large interface with gp120 glycans and regions of the gp120 inner domain not contacted by other bNAbs. By isolating additional 8ANC195 clonal variants, we identified a more potent variant, which may be valuable for therapeutic approaches using bNAb combinations with nonoverlapping epitopes.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






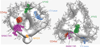
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

