Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis
- PMID: 24767987
- PMCID: PMC4049626
- DOI: 10.1016/j.celrep.2014.03.057
Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis
Abstract
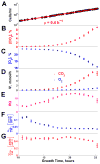
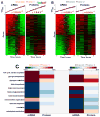
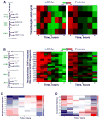
Fermenting glucose in the presence of enough oxygen to support respiration, known as aerobic glycolysis, is believed to maximize growth rate. We observed increasing aerobic glycolysis during exponential growth, suggesting additional physiological roles for aerobic glycolysis. We investigated such roles in yeast batch cultures by quantifying O2 consumption, CO2 production, amino acids, mRNAs, proteins, posttranslational modifications, and stress sensitivity in the course of nine doublings at constant rate. During this course, the cells support a constant biomass-production rate with decreasing rates of respiration and ATP production but also decrease their stress resistance. As the respiration rate decreases, so do the levels of enzymes catalyzing rate-determining reactions of the tricarboxylic-acid cycle (providing NADH for respiration) and of mitochondrial folate-mediated NADPH production (required for oxidative defense). The findings demonstrate that exponential growth can represent not a single metabolic/physiological state but a continuum of changing states and that aerobic glycolysis can reduce the energy demands associated with respiratory metabolism and stress survival.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Appling DR. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. The FASEB journal. 1991;5:2645–2651. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

